Research Article
Histopathologic Features of Endometrial Carcinoma with Fallopian Tube İnvolvement
Kabukcuoglu S* and Yildirim GD
Department of Pathology, University of Osmangazi School of Medicine, Eskisehir, Turkey
*Corresponding author: Kabukcuoglu S, Department of Pathology, University of Osmangazi School of Medicine, Eskisehir, Turkey
Published: 11 May, 2018
Cite this article as: Kabukcuoglu S, Yildirim GD.
Histopathologic Features of Endometrial
Carcinoma with Fallopian Tube
İnvolvement. Clin Oncol. 2018; 3: 1448.
Abstract
Objectives: Endometrial Carcinoma (EC) is the most common invasive neoplasm in female genital
tract. Endometrial cancer occurs in both premenopausal and postmenopausal women. In the large
series, 4% to 6% of the cases have diagnosed with the Stage III and Stage IV disease. The reccurences
are seen at the same frequency with endometrial cancer cases. Sampling of fallopian tube according
to Sectioning and Extensively Examining the Fimbrial End Protocol (SEE-FIM) and examination
of dissected lymph nodes and peritoneal washing fluid cytology are necessary for appropriate
histopathologic classification of patients. Recent reports indicate that up to 25% of patients with
clinically Staged Stage I disease have positive lymph nodes and these cases have microcystic,
elongated, and fragmanted glands (MELF). The aim of this study is to document the histopathologic
features of endometrial cancer cases with tubal involvement.
Methods: Endometrial cancer cases were retrieved from the files of the Pathology Department of
Osmangazi University Hospital between the November 2013 and February 2017.
Results: A total of 13 endometrial cancer cases with tubal involvement were identified. Positive
or suspicious peritoneal washing fluid was observed in three cases. There were three cases that
are under 60 years old with superficial myometrial invasion and these cases were associated with
endometriosis. In all those cases that are above 60 years old there were deep myometrial invasion
and 5 cases had cervical involvement. A total of 7 cases with cervical involvement had not been
associated with endometriosis. There was a significant inverse correlation between cervical
involvement and endometriosis in the EC cases (P<0.05). Endometriosis was associated with the
cases under 60 years old. This correlation was found to be significantly important (P<0.05). The
patients having immunohistochemical features of T cell factor/APC/β-cathenin pathway were
mostly under 60 years old and this finding was also statistically significant (P<0.05). Among this
group two patients had additional abnormalities, which are dual loss of MLH1 and PMS2, and
strong p53 positivity. In cases above 60 years old, PTEN inactivation, aging and loss of PAX8 were
detected by immunohistochemistry.
Conclusion: There is no specific immunohistochemical marker for discrimination of the spilling
tubal cells in tubal lumina or peritoneum with the low grade endometrial cancer cells. In this study
CK19, P16, WT-1, PAX-8 and calretinin immunohistochemistry were found as useful markers for
true classification.
Keywords: Senescence of fallopian tube; Calretinin signature; Endometrial carcinoma; Stage III; Myoinvasion pattern; Immunohistochemistry
Introduction
There is no single accepted follow-up strategy for patients with Endometrioid Endometrial Cancer (EEC). Early tumor reccurence may be observed at all stages of endometrial cancer [1]. Joehlin-Price et al. [2] reported 16 cases of lymph node metastases in 464 consecutive case of FIGO grade 1 EEC. After an average of 26 months follow-up 20 patients showed reccurences. In this group 45% of cases had isolated vaginal reccurences and 55% had extravaginal reccurences. In recent years, studies about myoinvasion patterns of EEC have pointed that lymphovascular space involvement mostly occurs in the Microcystic Elongated Fragmented pattern (MELF) [3-6]. Havrilesky et al. [7] reported 24 cases of Stage IIIA1, non-serous and FIGO grade 1-2 endometrial carcinoma. There were no reccurences among 12 cases receiving adjuvant treatment. Among the other 12 cases receiving no adjuvant therapy, one patient had experienced an extranodal abdominal recurrence at 6 years postoperatively. Hu et al. [8] demonstrated increased actin bundling protein fascin expression in tissue samples and cell cultures derived from ovarian cancer and in tissues of borderline and carcinomatous ovarian neoplasms and suggested that fascin could serve as an important prognostic factor for abnormal ovarian epithelial pathology. Prognostic role of fascin was also shown in EEC with MELF pattern [4]. In tumors, differentiated epithelial cells mostly retain the keratin patterns of their epithelial origin. Caspases cleave cytokeratins during apoptosis. As a result, apoptotic bodies and soluble keratin fragments enter into the lymphovascular space [9,10]. Malignant cells in peritoneal washings fluid may be the result of trans-tubal dissemination of the primary tumor. Stewart et al. [11] conducted a study with 226 high grade and 36 low grade endometrial cancer patients who underwent surgical staging. Among these cases 26% of high grade and 3% of low grade endometrial cancer cases had intraluminal tumor cells. The presence of fallopian tube metastases and intra-luminal tumor cells was strongly correlated with positive peritoneal fluid cytology, peritoneal metastases and lymph node metastases in high grade tumor subtypes. Positive peritoneal cytology is an independent risk-factor in Stage I/II endometrial cancer [12]. Positive peritoneal cytology is also highly predictive of prognosis and relapse patterns in Stage III endometrial cancer, and is correlated with higher reccurence rates in the paraaortic nodes and peritoneum [13]. Serous endometrial carcinomas may rarely be detected by peritoneal washing cytology [14]. Differential diagnosis of cancer cells in peritoneal cytology by using immunohistochemistry may not be possible in most of the FIGO grade I cancers. Most of the immunohistochemical markers such as p16, cytokeratins, estrogen receptor, progesteron receptor, p53, PAX8, p16 may stain both EEC cells and fallopian tube epithelium. The aim of the study is to detect fallopian tube metastasis and intraluminal tumor cells in endometrial cancer patients who underwent surgical staging by using SEE-FIM protocol and immunohistochemistry.
Table 1
Figure 1A
Figure 1A
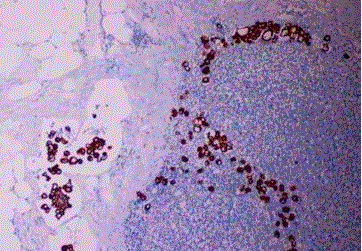
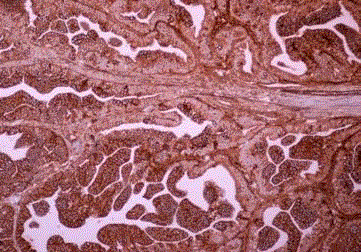
Endometrioid carcinoma, cytokeratin positive histiocyte-like
tumor cells are seen in the lymph node sinus (Case 2).
Figure 1B
Figure 1C
Figure 1C
Focal calretinin signature in tubal epitelium. Inflammatory exudate
did not stain with calretinin.
Materials and Methods
Patients who underwent total abdominal histerectomy, bilateral
salpingo-oophorectomy, bilateral pelvic paraaortic lymph node
dissection and infracolic omentectomy because of endometrial
carcinoma were examined by light microscopy. Patients' age, tumor
type, tumor grade, tumor localization, depth of tumor invasion,
cervical involvement, fallopian tube involvement, numbers of
reactive and metastatic lymph nodes and extrauterin or omental
histopathologic features and peritoneal washing fluid cytology
results, type of the surgical procedure were taken from computer
based patients' pathology reports at the Osmangazi University
Hospital between November 2014 to February 2016. A total of 13
Stage III and Stage IV EC patients with fallopian tube involvement
were found from the surgical pathology files. All of the tubes were
examined according the SEE-FIM protocol including intramural tuba
portion. All of the lesions of fallopian tubes and operation specimens
had been recorded in the reports.
Immunohistochemistry
All of the immunohistochemical stainings which were applied to
the EC cases had been recorded in computer based patients' pathology
reports. The immunohistochemical studies were performed using a
standart procedure on an automated immunostainer. Liquid rabbit
monoclonal CK19, P53, CDX2, β-cathenin, E-cadherin, estrogen
receptor, progesteron receptor, PAX-8, chromogranin were used
as primary antigen. WT-1, p16, calretinin, MLH1, PMS2, MSH2,
MSH6 were also used as primary antigens if they were necessary
for differential diagnosis. The primary antibody was replaced PBS
as a negative control. Diaminobenzidine was used as chromogen.
Finally, the sections counter stained with Mayer's hematoxylin, and
the sections were dehydrated, cleared and mounted. According to the
records MLH1, PMS2, MSH2, MSH6 applied to five cases ( Case no:
1, 2, 3, 4, 6) at the (Table 1).
Statistical analysis
Pearson chi square statistic was used for data analysis.
Table 2
Results
Clinical and histopathologic features of endometrial
cancer patients
The patients' ages ranged from 47 to 85 years (mean, 63 y).
Table 1 briefly describes the patients' age, histopathologic type of
the tumor, surgical Stage's, cervical involvement, and follow-up
of the patients. Table 2 briefly shows histopathologic type, specific
immunohistochemical features of the case, myoinvasion degree
and pattern, tubal, serosal and ovarian involvement, peritoneal
fluid cytology, metastatic and reactive lymph nodes. Eight patients
were grade 2. There was one dedifferentiated EC, one EC arising
adenofibroma, one MMMT, one EC with squamous differentiaton
and one EEC among the five grade 3 cases. One EC case with
mucinous differrentiation associated with granulosa cell tumor of
ovary and tubal endometriosis (Case 8). In 10 cases there were deep
myometrial invasions, in 3 cases there were superficial myometrial
invasions. Four cases were associated with endometriosis (patient 3,
8, 9, 10), among these cases three cases had superficial myometrial
invasion. There were 7 cases with cervical involvement and all of
these cases had deep myometrial invasions. There were no cases
associated with endometriosis among them. Above 60 years old all
of the patients' had deep myometrial invasion. There was no omental
involvement among the surgically Staged cases.
Immunohistochemistry results
Immunohistochemistry showed T cell factor/APC/β-cathenin
(wnt) pathway involvement in 8 cases. In these cases CDX2 or p63
positive squamous differentiation areas showed decreased estrogen
receptor, progesteron receptor and CK19 expression and this features
were compatible with β-cathenin mutation (Case 1, 6, 8, 11 ). Of these
cases one patient at Stage IV had dual MLH1 and PMS2 loss and was
associated with 27 metastatic lymph nodes (Case 6).
Focal or complete E-cadherin loss associated with decreased
estrogen receptor, progesteron receptor and CK19 expression were
compatible with E-cadherin gen (CDH1 ) mutation and were associated
with T cell/APC/β-cathenin pathway (Case 2, 3, 9, 10). One of these
cases had strong p53 positivity and associated with endometriosis
and omental vascular trombosis (Case 10). A 85 years old patient
had complete PAX-8 loss (Case 4). Dedifferentiated EC case showed
PTEN inactivation and weak p53 positivity (Case 5). Patient 7 was 75
years old and wild type p53 was observed. Decreased estrogen receptor
and progesteron receptor expressions were associated with negative
CDX2 and p16. CD10 and p16 expression were observed in endometrial
carcinoma arising adenofibroma (Case 12). MMMT was stained with
PAX8, desmin, myogenin (Case 13). There was a significant inverse
correlation between cervical involvement and endometriosis in EC
cases (P<0.05). Endometriosis was associated with the cases under
60 years old. This correlation was significantly important (P<0.05).
The patients having immunohistochemical features of T cell factor/
APC/β-cathenin pathway were mostly under 60 years old and this
finding was statistically significant (P<0.05).
Figure 2A
Figure 2A
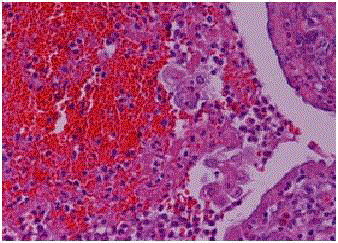
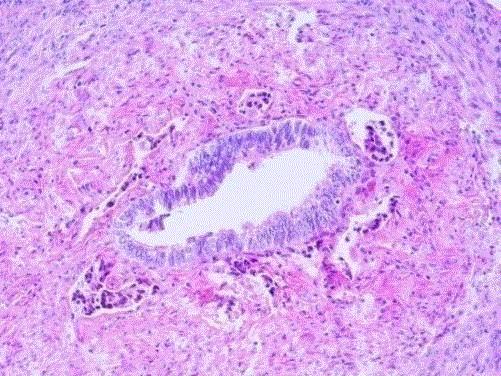
Histiocyte-like intra-luminal tumor cell groups in the fimbrial end
of the tuba. Duct-like appearance of tubal epithelium and muscle layer (Case 3).
Figure 2B
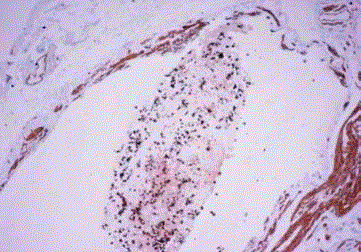
Figure 3
Figure 3
Proliferative glandular type tumor embolies in lympovascular space
around the intramural tuba segment (Case 4).
Figure 4
Figure 4
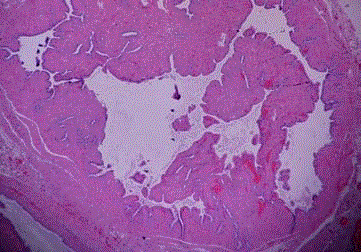
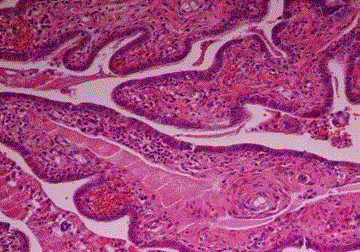
Endometrial carcinoma, dedifferentiated type. A few pleomorphic
tumor cells among inflammatory exudate in the tubal lumina (Case 5).
Figure 5
Figure 6
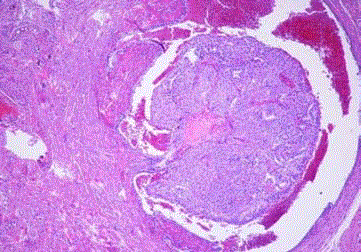
Figure 6
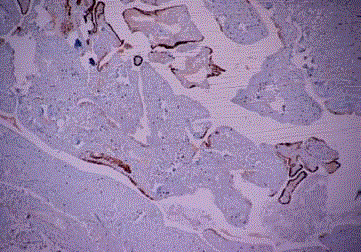
EEC with squamous differentiation, tumoral emboli is seen in tubal
vascular lumina. (CK19 immunohistochemistry, case 7).
Dıscussion
Endometrioid endometrial carcinoma has a good clinical outcome with low reccurence rate and metastasis. Prognostic factors for EEC are patient age, tumor grade, histological subtype, depth of myoinvasion, myoinvasion pattern, extrauterine extension and Lymph Node (LN) metastasis [6]. The patient's age is closely related to tumor types and histotypes. Endometrial carcinomas are divided into two types. Type I tumors are low grade endometrial carcinoma. Histopathologic prototype of type 2 tumors is serous carcinoma. Uterine serous carcinomas (USC) are extensively associated with p53 mutations and the often loss of the expression of the estrogen receptor [15]. In type I tumors mutations of PTEN, KRAS, PIK3CA and CTNNBI genes were frequently found and microsatellite instability coexisted in some cases. Loss of heterozygosity in cell cycle genes were also found in EEC [16]. Mixed and dedifferentiated endometrial carcinomas, clear cell carcinoma, malign mixed mullerian tumors and carcinomas arising from atypical polypoid adenomyomas, adenofibroma and endometrial polyps might be present in advanced stages and age [17]. In this study, T cell factor/APC/β-cathenin pathway associated immunohistochemical expression abnormality were detected in the 8 of 15 EEC patients. One of these cases coexisted with strong p53 expression (Case 10). Dual loss of MLH1 and PMS2 were detected in one patient of ECC who had defective T cell factor/ APC/β-cathenin pathway. In this case calretinin signature was observed in tubal epithelium (Case 6, Figure 5). PTEN inactivation with weak p53 expression and the loss of PAX8 expression coexisting with strong p16 staining were detected in two cases diagnosed as dedifferentiated EC and MMMT, respectively. Kommoss et al. [18] detected 32 cases of fallopian tube metastases associated with 161 USC cases by using WT-1 and p53 immunohistochemistry. In their series, 17 of 30 mucosal metastases resembled STIC-like features. Twelve cases of STIC-like features were accepted as USC metastases. Two cases were probably metastatic USC and one case had uncertain origin. Two cases were considered to be of primary tubal origin. Giordano et al. [14] detected one USC that involved an endometrial polyp which was associated with positive peritoneal washing cytology and with simultaneous carcinoma of tubal fimbria. P16 is a tumor supressor gene. Horre et al. [19] showed that p16 immunostaining was seen in endometrial tubal metaplasia. Simon et al. [20] suggested that the presence of typical and atypical tubal metaplasia did not increase the risk of developing endometrial hyperplasia and endometrial cancer in the long term follow-up by using Ki-67, p53 and TERT immunohistochemical staining. Similarly, I observed a patient p53 who had become negative when she underwent histerectomy because of simple atypical hyperplasia whereas there were 40% positive p53 staining in preoperative endometrial biopsy. Acute stress results in apoptosis and, while chronic stress results in DNA damage and aging [21, 22]. CD95 is a transmembrane glycoprotein which belongs to the nerve growth factor/tumor necrosis factor (TNF/NGF) receptor superfamily. When fascin was downregulated CD95 shows tumorigenic activity by a pathway involving JNK and c-JUN. CD95 promotes tumor growth [4,23-25]. Lu et al. [26] had made a model serine phosphorilation of p16 which resulted in arginine methylation by H202 for modulating cellular apoptosis and senescence. Doxorubicin induces apoptosis in cancer cells by similar mechanisms [27]. In this study, p16 expression in high grade endometrial cancer was observed in carcinoma arising in an adenofibroma (Case 12). Fallopian tube is an important passage for EEC and high grade endometrial cancer cells to peritoneum. Most of the immunohistochemical markers stain both EEC and tubal epithelium. When there are inflammatory exudates or the spilling tubal cells into the tubal lumina or peritoneum, it may not be possible to define tumoral cells by light microscopic examination. In the case it is neccesary to use immunohistochemical markers highlighting senescence of tubal epithelial cells. In cases of ageing and photoageing some common features such as enhancing p53 mutations, oxidative stress, malignant transformation and immortalization may be detected [28]. Similarly, it is recognised that 1,25D also exerts nongenomic actions by the activation of signalling molecules, such as phospolipase C and phospholipase A2 , phosphatidylinositol-3 kinase (PI3K) and p21 RAS, and rapid generation of second messengers (Ca2+, cyclic AMP, fatty acids and 3-phosphoinositides such as phosphatidylinositol 3,4,5 triphosphate). The activation of these molecules is associated with the activation of protein kinases, such as protein kinase A, src oncoprotein, mitogen activated protein kinases, protein kinase C and Ca2+ calmoduline kinase II. The nongenomic actions also include the opening of Ca2+ and Cl- channels [29]. Sakaguchi et al. [30] showed steroid receptor coactivator (SRC)- 3 mRNA expression correlated poor prognosis in endometrial cancer. The other studies had demonstrated that SRC-3 (CBP-interacting protein) had important role on carcinogenesis and metabolic pathways [31,32] One alpha, 25 dihydroxyvitamin D3 upregulates calcium binding proteins (CBP). Ovarian surface epithelium, stromal and thecal cells, follicular cysts, corpora lutea and rete ovary and endometrial stromal cells, histiocytes and fibroblasts show strong calretinin expression. Normal fallopian tube epithelium does not express calretinin [33]. Calretinin expression (calretinin signature) of fallopian tube epithelium may be associated with some endometriosis cases [34] and/or endometriosis associated with benign and malign neoplasms including endometrial carcinoma. As a result, calretinin is also a useful marker for differentiating intra-luminal or peritoneal tumor cells from transformed tubal cells. Eucher et al. [6] reported a case who had no myometrial invasion but had a focus of metastatic carcinoma in the fallopian tube. Han et al. [35] reported that reduced E-cadherin expression was associated with MELF pattern of MI, and these cases may have occult lymph node metastasis even when they were at Stage I. Hertel et al. [36] had made differential diagnosis between histiocyte-like cancer cells and histiocytes in the lymph nodes of ECC patients by using CD68, calretinin, cytokeratin and epithelial membrane antigen immunohistochemistry. Despite of the differences in MI patterns and degree of MI, the first three cases in the study group had E-cadherin loss or β-cathenin mutation and they had Stage III disease at the operation time (Table 2, Figure 1A, 1B, 1C and Figure 2A, 2B). The histopathologic differences among three cases are the presence of inflammatory exudate in the tubal lumina and calretinin signature in tubal epithelium and p16 staining of tumor cells. Further studies are necessary to better clarify the effect of MI pattern on the Stage of ECC and its relation with calretinin signature of fallopian tube [37]. Our study showed that p16, CK19, PAX-8, WT-1 and Calretinin immunohistochemistry are useful for discrimination of tumor cells among the spilling tubal or peritoneal fluid cells.
References
- Crispens MA, Schorge JO, Schaffer JJ, Halvarson LM, Hoffman BL, Bradshaw KD, et al. Williams Gynecology. McGraw Hill Medical. 2008;687.
- Joehlin-Price AS, McHugh KE, Stephens JA, Zaibo L, Backes FJ, Cohn DE, et al. The microcystic, elongated, and fragmented (MELF) pattern of invasion: A single institution report of 464 consecutive FIGO Grade 1 endometrial endometrioid adenocarcinomas. Am J Surg Pathol. 2017;41(1):49-55.
- Murray SK, Young RH, Scully RE. Unusual epithelial and stromal changes in myoinvasive endometrioid adenocarcinoma: a study of their frequency, associated diagnostic problems, and prognostic significance. Int J Gynecol Pathol. 2003;22(4);324-33.
- Stewart CJ, Crook ML, Manso L. Fascin expression in low-grade uterine endometrioid adenocarcinoma: correlation with microcystic, elongated and fragmented (MELF)-type alteration at the deep invasive margin. Histopathology. 2011;59(1):73-80.
- Pavlakis K, Messini I, Vrekoussis T, Panoskaltsis T, Chrysanthakis D, Yiannou P, et al. MELF invasion in endometrial cancer as a risk factor for lymph node metastasis. Histopathology. 2011;58(6):966-73.
- Euscher E, Fox P, Basset R, Al-Ghawi H, Ali-Fehmi R, Barbuto D, et al. The pattern of myometrial invasion as a predictor of lymph node metastasis or extrauterine disease in low grade endometrial carcinoma. Am J Surg Pathol. 2013;37(11):1728-36.
- Havrilesky LJ, Secord AA, O'Malley DM, Broadwater G, Bae-Jump V, Cohn DE, et al. Multicenter analysis of recurrence and survival in Stage IIIA endometrial cancer. Gynecologic Oncology. 2009;114(2):279-83.
- Hu W, McCrea PD, Deavers M, Kavanagh JJ, Kudelka AP, Verschraegen CF. Increased expression of fascin, motility associated protein, in cell cultures derived from ovarian cancer and in borderline and carcinomatous ovarian tumors. Clin Exp Metastasis. 2000;18(1):83-8.
- Lee JC, Schickling O, Stegh AH, Oshima RG, Dinsdale D, Cohen GM, et al. DEDD regulates degradation of intermediate filaments during apoptosis. J Cell Biol. 2002;158(6);1051-66.
- Shah YM, Basrur V, Rowan BG. Selective estrogen receptor modulator regulated proteins in endometrial cancer cells. Mol Cell Endocrinol. 2004;219(2):127-39.
- Stewart CJ, Doherty DA, Havlat M, Koay MH, Leung YC, Naran A, et al. Transtubal spread of endometrial carcinoma: correlation of intraluminal tumour cells with tumour grade, peritoneal fluid cytology, and extrauteruterine metastasis. Pathology. 2013;45(4):382-7.
- Garg G, Gao F, Wright JD, Hagemann AR, Mutch DG, Powell MA. Positive peritoneal cytology is an independent risk- factor in early Stage endometrial cancer. Gynecologic Oncology. 2012;128(1):77-82.
- Milgrom SA, Kolmeier MA, Abu-Rustum NR, Makker V, Gardner GJ, Barakat RR, et al. Positive peritoneal cytology is highly predictive of prognosis and relapse patterns in Stage III (FIGO 2009) endometrial cancer. Gynecologic Oncology. 2013;130(1):49-53.
- Giordano G, Varotti E, Brigati F, Beretta R. The value of peritoneal washing cytology during intra-abdominal surgery for female genital tract neoplasms. Clin Genitourinary Can. 2014;12(3):e95-101.
- Hoang LN, Kinloch MA, Leo JM, Grondin K, Lee C, Ewanowich C, et al. Interobserver agreement in endometrial carcinoma histotype diagnosis varies depending on the cancer genome atlas (TCGA)-based molecular subgroup. Am J Surg Pathol. 2017;41(2):245-52.
- Velasco A, Pallares J, Santacana M, Yeramian A, Dolcet X, Eritja N, et al. Loss of heterozygosity in endometrial carcinoma. Int J Gynecol Pathol. 2008;27(3):305-17.
- Soslow RA. High grade endometrial carcinomas-stategies for typing. Histopathology. 2013;62(1):89-110.
- Kommos F, Farugi A, Gilks B, Leen SL, Singh N, Wilkinson N, et al. Uterine serous carcinomas frequently metastasize to the fallopian tube and can mimic serous tubal intraepitelial carcinoma. Am J Surg Pathol. 2017;41(2):161-70.
- Horree N, Heintz APM, Sie-Go DMDS, vanDiest PJ. P16 is consistently expressed in endometrial tubal metaplasia. Cellular Oncol. 2007;29(1):37- 45.
- Simon RA, Peng SL, Liu F, Quddus MR, Zhang C, Steinhoff MM, et al. Tubal metaplasia of the endometrium with cytologic atypia: analysis of p53, Ki-67, TERT, and long term follow-up. Mod Pathol. 2011;24(9):1254- 61.
- Carasco-Garcia E, Moreno M, Moreno-Cugnon L, Matheu A. Increased Arf/p53 activity in stem cells, aging and cancer. Aging Cell. 2017;16(2):219- 25.
- Wang G, Fu Y, Hu F, Lan J, Xu F, Yang X, et al. Loss of BRG1 induces CRC cell senescence by regulating p53/p21 pathway. Cell Death and Disease. 2017;8:E2607.
- Bodey B, Bodey BJr, Siegel SE, Kaiser HE. Fas (Apo-1, CD95) receptor expression in childhood astrocytomas. Is it a marker of the major apoptotic pathway or a signaling receptor for immune escape of neoplastic cells?'. In vivo. 1999;13(4):357-73.
- Chen L, Park SM, Tumanov AV, Hau A, Sawada K, Feig C, et al. CD95/Fas promotes tumour growth. Nature. 2010;465(7297):492-6.
- Reno EM, Haughian JM, Jackson TA, Thorne AM, Bradford AP. c-Jun N-terminal kinase regulates apoptosis in endometrial cancer cells. Apoptosis. 2009;14(6):809-20.
- Lu Y, Ma W, Li Z, Lu J, Wang X. The interplay between p16 serine phosphorylation and arginine methylation determines its function in modulating cellular apoptosis and senescence. Scientific Reports. 2017;7:41390.
- Mohammad N, Singh SV, Malvi P, Chaube B, Athavale D, Vanuopadath Mm, et al. Strategy to enhance efficacy of doxorubicin in solid tumor cells by methy-β-cyclodextrin: Involvement of p53 and Fas receptor ligand complex. Scientific Reports. 2015;7(5):11853.
- Smith KJ, Germain M, Skelton H. Telomeres and telomerase in ageing and cancer; with emphasis on cutaneous disease. J Cutan Pathol. 2000;27(1):2- 18.
- Hii CS, Ferrante A. The non-genomic actions of vitamin D. Nutrients. 2016;8(3):135.
- Sakaguchi H, Fujimoto J, Sun W, Tamaya T. Clinical implications of steroid receptor coactivator (SRC)-3 in uterine endometrial cancer. J Steroid Biochem Mol Biol. 2007;104(3-5):237-40.
- Gang M, Ren Y, Wang K, Janjun H. Src-3 has a role in cancer other than as a nuclear receptor coactivator. Int J Biol Sci. 2011;7(5):664-72.
- York B, Sagen JV, Tsimelzon A, Louet JF, Chopra AR, Reineke EL, et al. Research resource: Tissue- and pathway-specific metabolomic profiles of the steroid receptor coactivator (SRC) family. Mol Endocrinol. 2013;27(2):366-80.
- Banet N, Kurman RJ. Two types of ovarian cortical inclusion cysts: Proposed origin and possible role in ovarian serous carcinogenesis. Int J Surg Pathol. 2015;34(1):3-8.
- Barcena de Arellano ML, Munch S, Arnold J, Helbig S, Scheneider A, Mechsner S. Calcium-binding protein expression in peritoneal endometriosis-associated nerve fibers. Eur J Pain. 2013;17(10):1425-37.
- Han G, Lim D, Leitao MM, Abu-Rustum NR, Soslow RA. Histological features associated with occult lymph node metastasis in FIGO clinical Stage I, grade 1 endometrioid carcinoma. Histopathology. 2014;64(3):389- 98.
- Hertel JD, Huettner PC, Pfeifer JD. Lymphovascular space invasion in microcystic elongated and fragmented (MELF)-pattern well-differentiated endometrioid adenocarcinoma is associated with a higher rate of lymph node metastasis. Int J Gynecol Pathol. 2014;33(2):127-34.
- Zaino RJ. Unusual patterns of endometrial carcinoma including MELF and its relation to epithelial mesenchymal transition. Int J Gynecol Pathol. 2014;33(4);357-64.