Case Report
Progressive Neurotoxicity after Intrathecal Methotrexate and Cytarabine in a Child with Acute Myeloid Leukemia
Arushi Gahlot Saini1, Jitendra Kumar Sahu1, Sonali Mahapatra1, Deepak Bansal2, Paramjeet Singh2, Pratibha Singhi1*
1Department of Pediatrics, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, India
2Department of Radiodiagnosis, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, India
*Corresponding author: Pratibha Singhi, Department of Pediatrics, Chief Pediatric Neurology and Neurodevelopment, Advanced Pediatrics Centre, Post Graduate Institute of Medical Education and Research, India
Published: 02 Jan, 2018
Cite this article as: Saini AG, Sahu JK, Mahapatra
S, Bansal D, Singh P, Singhi P.
Progressive Neurotoxicity after
Intrathecal Methotrexate and
Cytarabine in a Child with Acute
Myeloid Leukemia. Clin Oncol. 2018;
3: 1392.
Abstract
Background: Progressive encephalomyelitis with fatal course is an uncommon complication of
intrathecal chemotherapy in children with acute myeloid leukemia
Aims: We describe the clinical and radiological spectrum of progressive neurotoxicity after
combined intrathecal use of methotrexate and cytarabine in a child with acute myeloid leukemia
Methods: A 10-year-old boy suffering from acute myeloid leukemia developed acute-onset,
progressive ascending myelitis followed by encephalopathy after triple intrathecal chemotherapy.
He underwent detailed neurological assessments and relevant laboratory and radiological
investigations. Intravenous antimicrobials, methyl prednisolone pulse therapy and megavitamins
were administered.
Results: Examination showed high-frequency horizontal nystagmus, diffuse meningismus,
bilaterally symmetrical paraplegia, hypotonia, areflexia and flexor Babinski response in the lower
limbs, saddle anaesthesia and urinary retention. Cerebrospinal fluid analysis showed 400 cells
(95% polymorphonuclear cells, no blasts). Magnetic resonance imaging of spine showed long-tract
myelitis with caudal nerve-root enhancement. Nerve-conduction study showed severe sensorymotor
polyneuropathy involving lower limbs. The clinical course evolved from an acute, reversible
chemical arachnoiditis to cauda-equina syndrome to a progressive, treatment-refractory, devastating
encephalomyelitis over three weeks. He finally succumbed to nosocomial sepsis and cardiac arrest
Conclusion: Progressive neurotoxicity following intrathecal chemotherapy, especially with
combination of methotrexate and cytarabine needs consideration in pediatric patients of leukemia.
Early identification of evolving neurological signs may help in the initiation of early salvage therapy.
Keywords: Neurotoxicity; Intrathecal; Methotrexate; Cytarabine; Cauda-Equina;
Encephalomyelitis
Introduction
Intrathecal chemotherapy with methotrexate and cytosine-arabinoside (cytarabine) is an established modality for central nervous system prophylaxis in pediatric acute myeloid leukemia (AML). Neurotoxicity is a common complication with either drug, however fatal course is exceptional and usually associated with combined use or cumulative doses of methotrexate and cyarabine. We describe the gradually progressive, treatment-refractory, fatal neurotoxicity from combined intrathecal chemotherapy with methotrexate and cytarabine in a 10-year-old boy suffering from AML. Identification of evolving neurological signs is important in anticipating the cause and course of illness. Such atypical progressive neurotoxicity in a single patient is rarely seen with pediatric AML and merits attention.
Case Report
A 10-year-old boy suffering from AML (stage 2) developed acute-onset, non-projectile vomiting
and intermittent, dull headache four hours after triple intrathecal chemotherapy (methotrexate
12 mg, cytarabine 30 mg and hydrocortisone 12.5 mg). Within the next 24 hours, he developed
neck stiffness and back pain. Lumbar puncture prior to the institution of intrathecal chemotherapy
showed normal glucose and proteins, and no cellular response or blast cells in the cerebrospinal
fluid (CSF). He was given supportive treatment for probable chemical
meningitis. On second day, parents noticed difficulty in bearing weight
on the lower limbs and requirement of support for ambulation. There
was no weakness in the trunk or upper limbs. On the third day, he
developed moderate-grade intermittent fever and acute-onset urinary
retention requiring hospitalization. There was no history suggestive
of encephalopathy, seizures, behavioural abnormalities, cranial nerve
palsies, vision impairment, extrapyramidal movements or sensory
symptoms. The patient had received his first chemotherapy course
including the same triple intrathecal drugs one month back. There
were no blasts in the CSF or bone-marrow.
Examination at admission showed intermittent, gazeindependent,
high-frequency horizontal nystagmus, bilaterally
symmetrical weakness (Medical Research Council grade 3/5),
hypotonia, absent muscle stretch reflexes and flexor Babinski sign
in the lower limbs, saddle anaesthesia, urinary retention, diffuse
meningismus with neck rigidity and straight-leg-raising positive at
60°. Higher mental functions, cranial nerve including fundi and upper
limb examination was normal. There was no cerebellar ataxia, extra
pyramidal or sensory signs, local spine tenderness or hematoma.
Clinical syndrome of drug-induced aseptic meningitis with caudaequina
syndrome was considered. CSF showed 400 cells (95%
polymorph nuclear cells, no blasts), protein 0.73 g/L, glucose 3.16
mmol/L (blood glucose 5.83 mmol/L) and negative gram-stain and
culture. Haemoglobin was 100 g/L, total leucocyte count 11.7x109/L
(78% neutrophils, 12% lymphocytes, 10% monocytes), platelets 348
x109/L, prothrombin index 82% and C-reactive-protein was 0.10 g/L
(normal <0.08 g/L). Contrast-enhanced magnetic resonance imaging
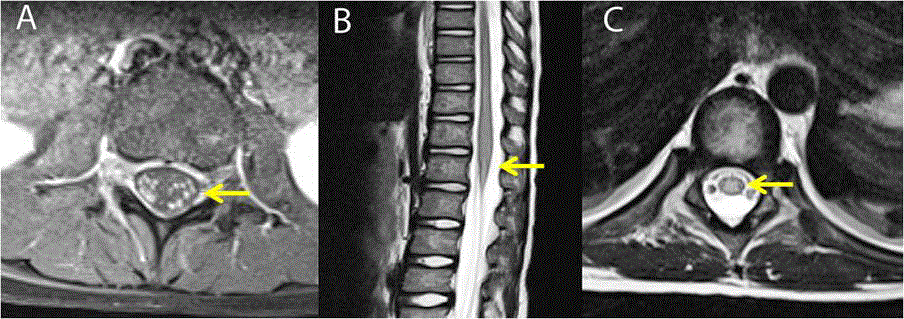
(MRI) spine showed caudal nerve-root enhancement (Figure 1A) and
meningeal enhancement at the base of the brain. In view of persisting
fever and meningeal signs, he was administered intravenous
antimicrobials (vancomycin 50 mg/kg/day q8hourly, meropenem 90
mg/kg/day q6hourly, acyclovir 30 mg/kg/day q8hourly) for possible
bacterial meningitis. Fever and meningeal signs subsided within next
48 hours. On sixth day, the patient developed intermittent, irrelevant
talking and drowsiness associated with bilateral papilloedema.
Contrast-enhanced computed tomography brain scan was normal.
He improved with intravenous 3% hypertonic saline infusion. Repeat
lumbar CSF showed 60 cells (70% polymorphonuclear cells), glucose
3.22 mmol/L (blood glucose 6 mmol/L) and protein 0.91 g/dL. No
organism was identified on gram-stain and culture, triple-antigen
testing, viral polymerase chain reaction studies, and mycoplasma
serology. From seventh day, weakness gradually progressed to
flaccid, symmetric paraplegia (Medical Research Council grade
1/5) with ascending truncal and respiratory weakness, mild upper
limb hypotonia, absent joint-position and vibration sense below
thoracic cord, and persisting urinary retention. Clinical possibility
of ascending encephalomyelitis due to drug-associated neurotoxicity
or infection was considered. Repeat contrast-enhanced MRI-spine
showed long-tract myelitis with caudal nerve-root enhancement
(Figure 1B-C). Nerve-conduction study on tenth day showed
severe sensory-motor polyneuropathy of uncharacterized nature
involving the lower limbs alone. Intravenous pulse methylprednisol
one (25mg/kg/day q24hourly for 5 days); oral folinic acid (60 mg/
day), S-adenosyl methionine (200 mg/day), injection aminophylline
(70 mg) and injection B12 (1000 μg/day) were added. There was
gradual progression of encephalopathy and weakness into flaccid
quadriparesis and respiratory failure requiring manual ventilation.
He finally succumbed to nosocomial sepsis and cardiac arrest on day
22 and could not be revived. Parents denied autopsy on the child.
Figure 1
Figure 1
MRI spine: (A) Contrast-enhanced T1-weighted axial sections showing cauda-equina nerve-root enhancement (arrow), (B) T2-weighted sagittal and (C)
axial sections showing central hyperintensity (arrow) in thoraco-lumbar cord suggestive of myelitis.
Discussion
Our case highlights the clinical and radiological evolution of
intrathecal chemotherapy-associated neurotoxicity. It progressed
from drug-induced, reversible chemical arachnoiditis, to lumbosacral
radiculopathy manifesting as cauda-equina syndrome, to
irreversible ascending encephalomyelitis in the same patient. Similar
fatal radiculo-encephalomyelitis following intrathecal chemotherapy
has been scarcely reported in children and warrants multi-speciality
care [1].
Infectious meningitis and leukemic meningeal infiltration
constitute important diagnostic considerations and therapeutic
priority in such immune-compromised patients. The presence of
fever, meningismus and CSF polymorph nuclear pleiocytosis may
mimic bacterial infection. However, bacterial and viral studies from
CSF did not identify any infectious agent in our patient. Blasts cells
were repeatedly absent from the CSF. Absence of compressive lesion
on neuroimaging, close temporal relationship and lumbar puncture
site via L3/L4 vertebral level (which is free of spinal-cord), support
that ascending myelitis in our patient was related to intrathecal
administration of methotrexate and cytarabine rather than traumatic
or chemical meningitis. The commoner causes of intrathecal druginduced
neurotoxicity are accidental intrathecal administration of
the drug instead of intravenous route or miscalculation of intrathecal
dose leading to overdosing. There causes were not plausible in our
patient as he received a pre-calculated and monitored intrathecal
dose. Additionally, cytarabine-induced aseptic meningitis may show
600-1400 polymorph nuclear cells/mm3 and upto 2g/L proteins
in the CSF, possibly due to drug-hypersensitivity or immunologic
dysregulation [2]. Whether transverse myelitis is an extreme
expression of drug-induced aseptic meningitis remains conjectural.
Both cytarabine and methotrexate are known to cause acute
leukoencephalopathy in children and are generally associated with
white-matter hypodensities on computed tomography and areas
of diffusion-restriction and T2-hyperintensity on MRI-scan of
the brain [3]. However, our patient interestingly had normal MRI
scan despite worsening encephalopathy and transient features of
raised intracranial pressure. This lack of clear correlation between
radiological and clinical manifestations makes the diagnosis of “acute
leukoencephalopathy” difficult in these children. The persistent
nystagmus in our patient can also be attributed to the prominent
neurotoxic effect of cytarabine on the cerebellum [4].
Cauda-equina syndrome due to bilateral lumbo-sacral
polyradiculopathy after intrathecal methotrexate and cytarabine has
been less commonly described.5 Partial or completely absent motor
responses in lower limbs with sparing of upper limb motor and
sensory responses as seen in our case may indicate central damage to
the spinal cord or motor roots [5]. The absence of sensory response
in our patient could be due to technical difficulties in eliciting sural
responses in patients on manual ventilation.
Possible mechanisms of methotrexate-induced
leukoencephalopathy include drug-associated alterations in the
folate and methyl-transfer pathways in CSF and elevated adenosine
levels [6]. In the absence of established treatment guidelines
for methotrexate neurotoxicity, there are anecdotal reports of
improvement with systemic folinic-acid and competitive adenosine
antagonist aminophylline [7]. Our patient remained treatmentrefractory
despite the use of these rescue medications and intravenous
corticosteroid therapy.
Conclusion
Progressive neurotoxicity following intrathecal chemotherapy, especially with combination of methotrexate and cytarabine needs consideration in pediatric patients of AML. Early identification of evolving neurological signs may help in the initiation of early salvage therapy. Awareness regarding these manifestations is important for both the pediatric neurologists and hemato-oncologists.
References
- Brock S and Jennings HR. Fatal acute encephalomyelitis after a single dose of intrathecal methotrexate. Pharmacotherapy. 2004; 24(5): 673-6.
- van den Berg H, van der Flier M, van de Wetering MD. Cytarabine-induced aseptic meningitis. Leukemia 2001; 15: 697-9.
- Mahoney DH Jr, Shuster JJ, Nitschke R, Lauer SJ, Steuber CP, Winick N, et al. Acute neurotoxicity in children with B-precursor acute lymphoid leukemia: an association with intermediate-dose intravenous methotrexate and intrathecal triple therapy--a Pediatric Oncology Group study. J Clin Oncol 1998; 16: 1712-22.
- Soffietti R, Trevisan E and Ruda R. Neurologic complications of chemotherapy and other newer and experimental approaches. Handb Clin Neurol. 2014; 121: 1199-218.
- Park S, Kang JI, Bang H, Kim BR and Lee J. A case of the cauda equina syndrome associated with the intrathecal chemotherapy in a patient with primary central nervous system lymphoma. Ann Rehabil Med 2013; 37: 420-5.
- Vezmar S, Schusseler P, Becker A, Bode U and Jaehde U. Methotrexate-associated alterations of the folate and methyl-transfer pathway in the CSF of ALL patients with and without symptoms of neurotoxicity. Pediatr Blood Cancer 2009; 52: 26-32.
- Bernini JC, Fort DW, Griener JC. Aminophylline for methotrexate-induced neurotoxicity. Lancet. 1995; 345: 544-7.