Case Report
The Role of Somatostatin-Receptor Scintigraphy in the Diagnosis and Staging of Pulmonary Carcinoid Tumors
Walid Abu Arab1,2*, Yves Collin1, Marco Sirois1, Marc-André Levasseur3 and Nancy Paquet3
1Department of Medicine, University of Sherbrooke, Canada
2Department of Cardiothoracic Surgery, University of Alexandria, Egypt
3Department of Nuclear Medicine, University of Sherbrooke, Canada
*Corresponding author: Walid Abu Arab, Department of Medicine, University of Sherbrooke, 2001 avenue Nord, Fleurimont, J1H 5N4, Sherbrooke Québec, Canada
Published: 02 Jan, 2018
Cite this article as: Arab WA, Collin Y, Sirois M,
Levasseur M-A, Paquet N. The Role
of Somatostatin-Receptor Scintigraphy
in the Diagnosis and Staging of
Pulmonary Carcinoid Tumors. Clin
Oncol. 2018; 3: 1391.
Abstract
Background: Carcinoid tumors are rare low grade neuroendocrine tumors which rarely affect the
lung. Somatostatin - receptor scintigraphy (Octreo-scan) can be helpful in the diagnosis and staging
when the tumor is located in the gastro-intestinal tract (GI-tract). However, there is a few data in
the literature regarding its role in the diagnosis of pulmonary carcinoids. The aim of this study
is to assess the clinical utility of Somatostatin Receptor Scintigraphy (SRS) [OctreoScan®*] in the
diagnosis and staging of pulmonary carcinoids and its role in the treatment plan of the patients.
Methods: This is a retrospective clinical study that included the patients with pulmonary carcinoid
tumors who underwent lung resection at Centre HospitalierUniversitaire de Sherbrooke (CHUS),
Canada between 2004 and 2014. The files of those patients have been reviewed. Data collected
included the demographic data, methods of diagnosis, pathology, staging work-up as well as costs
of the effectuated investigations. Statistical analysis of was performed using Microsoft Excel 2007.
Results: Sixteen patients were included in this study. SRS was performed for eight of them, and it
was positive in seven patients (87% sensitivity). On contrast, Positive Emission Tomography (PETscan)
was found to have 76% sensitivity. The cost was 1100 CAN$ for PET-scan and 2000 CAN$ for
SRS. Radiation dose equivalence were comparable for both exams.
Conclusions: The SRS is a valuable method to detect and stage pulmonary carcinoid tumors. We
believe that it should be the method of choice for a diagnosis and staging when pulmonary carcinoid
tumor is suspected.
Keywords: Lung Cancer; Diagnosis of carcionoid; Neuroendocrine; Neuroendocrine tumor;
Positron Emission Tomography PET; Octreoscan; SRS
Introduction
Neuroendocrine tumors (NET) are tumors that originate from neural crest cells throughout the body. Carcinoid tumors are low grade malignant NETs with metastatic potential. Symptoms that are caused by carcinoid tumors are due to hormonal excess, local tumor growth, or metastasis. Surgical resection is the curative treatment for the localized disease [1-3]. Some authors classified pulmonary NETs into four categories: 1) typical carcinoid (also called bronchial carcinoid tumor, Kulchitsky cell carcinoma (KCC)-I); 2) atypical carcinoid (also called well-differentiated neuroendocrine carcinoma, KCC-II); 3) intermediate small cell neuroendocrine carcinoma; and 4) small cell carcinoma (KCCIII). Another proposed classification includes three categories of lung NETs: benign (typical carcinoid), low-grade malignant (atypical carcinoid), and high-grade malignant (poorly differentiated carcinoma of the large cell or small cell type) [2-4]. Atypical carcinoid tumors metastasize more commonly to the regional lymph nodes. The overall 5-year survival for atypical carcinoid tumors ranges between 40 to 69 % versus 87 to 100 % for the typical carcinoids [5]. NET cells can synthesize and secrete a variety of physiologically active peptides that are able to generate different clinical symptoms. The most common symptoms include flushing and diarrhea, which result from synergistic interactions between 5-HTP (5- Hydroxitryptamine) metabolites, kinins, and prostaglandins. This is known as carcinoid syndrome [1-3]. Carcinoid syndrome is rarely encountered in pulmonary carcinoid tumors [5-7]. The common feature for the majority of NETs is the expression of somatostatin peptide receptors (SSRs). This characteristic feature is employed for diagnostic imaging, using a radio-labeled somatostatin analog (Indium-111 octreotide). Somatostatine receptor scintigraphy could be helpful to assess the extent of the tumor spread in order to evaluate proper surgical treatment. Kwekkeboom et al. [8] documented that the combination of somatostatin receptor scintigraphyand other diagnostic tools can improve the staging process of the carcinoid tumors.Somatostatin-receptor scintigraphy (SRS; Octreoscan®, Mallinckrodt, Petten, The Netherlands) is performed by injecting a dose of radioisotope such as (111In- DTPA0) octreotide or (111In-pentetreotide) that could bind to the two most prevalent somatostatin receptors found on NETs (sst2 and sst5); this allows visualization and localization of NETs [9,10]. The use of SRS could be an option for detection and staging of pulmonary NETs. Nevertheless, SRS is currently rarely used for this purpose. We hypothesized that it should have a more important role in the workup for patients who have these type of tumors.
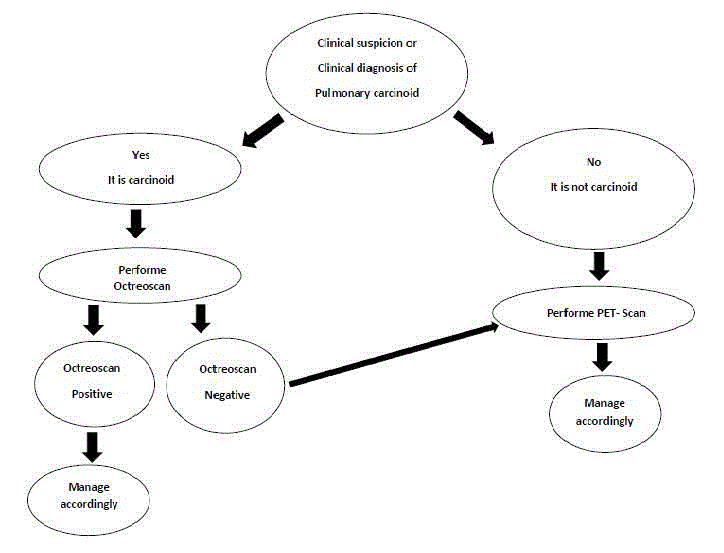
Figure 1
Table 1
Table 2
Table 3
Patients and Methods
A retrospective study protocol was approved by the Ethics Committee of the Centre de Recherche Étienne-Lebel at Centre HospitalierUniversitaire de Sherbrooke (CHUS), Canada. All patients with confirmed pulmonary carcinoid tumor who were operated at our center during the period between 2004 and 2012 were included in this study. The patients’ files were systematically reviewed. Data concerning Patients’ age, sex, respiratory function test, clinical presentation, pathology, diagnostic methods as well as their costs and their radiation doses were obtained. The results were arranged into three groups according to whether an SRS was performed and was found positive (Group I); SRS was done and was found negative (Group II); or the SRS was not done at all (Group III).
Results
Sixteen patients (37% male, 63% female) were included in this
study. Age ranged between 37-78 years with a mean of 59 years (Table
1). Three patients were smokers. Eight patients were asymptomatic
(50%). Seven patients (43%) presented with pulmonary symptoms.
The most frequent symptoms were cough and hemoptysis; while
Cushing’s syndrome was met with in one patient (6.25%) Pathological
diagnosis was obtained through bronchoscopy in 37.5% of patients
and through definitive surgery in 37.5% of them. Trans-thoracic
needle biopsy was used as a diagnostic method in 25% of patients.
Typical carcinoids tumors were found in 56% of the subjects while
atypical carcinoids were found in the remaining 44% of patients.
Five patients of sixteen patients (31.2%) had mediastinal lymph node
metastasis.
Sensitivity
Eight patients out of the sixteen had a SRS beside PET-Scan. SRS
was positive in seven patients (87.5%) while PET-Scan was positive
in 78.6% of patients (11 patients) (Table 2). In the group 1 (SRS done
and positive) (7 patients), only 57% of them had positive PET-scan
(Table 3). Therefore, PET-scan missed 43% of the tumors.
Comparison between Octreoscan and PET-scan positivity
Octreoscan was positive in 43% of male patients and in 57%
female patients. Meanwhile, PET-Scan was positive in 27% of male
patients. Lung function was comparable in both groups. Metastatic
adenopathies were found in 43% of positive Octreoscan at final
pathology, while it was found to be 27% with the PET-Scan.
Radiation level
Mean radioactivity emission was 350.5 ±47.6 MBq for PETScan
and 134.14 ± 26.7 MBq for SRS. When corrected for dose
equivalencies, emission for PET- scan was 6.7 mSv ± 0.9 while it was
7.3 ± 1.6 mSv for SRS.
Costs
The cost for the investigations in our center (CHUS-Canada) was
estimated to be 1965.00CAN$ for SRS and 1160.00CAN$ for PETscan.
A quick review of the literature revealed a cost of 20 000US$ for
SRS and 11 000US$ for PET-scan in the United States of America.
Discussion
The overall sensitivity of SRS in this study was slightly higher than that of PET-scan, which is consistent with the published data in the literature [11]. This is explained by the fact that while PETscan relies on metabolic activity for imaging purposes; which is low in carcinoids; SRS relies on somatostatin receptors which are found in more than 80% of carcinoid tumors [9,12]. Moreover, PET-scan was only positive in 57% of patients in whom SRS was also positive. This result led to the conclusion that when a diagnosis of pulmonary carcinoid tumor is confirmed or is highly suspected, SRS could be the imaging modality of choice for localization and staging purposes. Since the radiation equivalence of both exams is comparable, patients should not suffer from that change of attitude. Furthermore, even though SRS is more costly, we believe that; by using a single workup exam instead of two; as it is actually the case when PET scan is negative; the cost effectiveness of SRS could be better. Nevertheless, SRS is not the only trustable modality as it is shown by the one negative result in our series. Since PET-scan came out positive in that particular case, we think that PET-scan imaging still has place in the work-up of carcinoid tumors if SRS is negative. Since we only reviewed cases of pulmonary carcinoids, we still recommend PETscan as a primordial imaging modality in the diagnosis, staging and pre-operative planning of other types of lung nodules. We propose here an algorithm to clearly state the role of SRS in the management of pulmonary carcinoids (Figure 1). It is interesting to note that the proportion of smokers in our study was very low, which is consistent with the fact that smoking only has a marginal effect on the development of lung carcinoids [13]. Even with the small number of patients that were included in our study, we some tendencies in the group 1 were detected compared to positive PET-scan. Namely, male proportion that was higher. Final pathology reports showed more typical carcinoids but also more positive adenopathies. These results seem contradictive since typical carcinoids are usually less aggressive and have a lesser tendency to metastasize. It was believed that further studies should be performed to verify the predictors of sensibility for both SRS and PET-scan in patients with pulmonary carcinoids. This study has few limitations. First one is that the study is retrospective one and non-blinded but since we included every patient operated for lung carcinoid tumor during the period of the study, we don’t think that the retrospective nature of the work induced any bias. Second, the sample size is small, a fact inherent with research performed for rare diseases as pulmonary carcinoid tumors. This may led to statistical bias to some extent. Consequent to the previously mentioned raisons, it is recommended to perform further randomized prospective studies in the future to better clarify the results of our group.
Conclusion
The SRS is a diagnostic tool that can be helpful in pulmonary carcinoids. It can help to better localize and to precise the staging of the tumor. SRS could become the imaging modality of choice when suspecting a diagnosis of pulmonary carcinoid tumor. Hence, it could be reserved as a second line imaging modality. Further clinical randomized studies are necessary to establish the exact role of SRS in pulmonary carcinoid tumors.
References
- Beasley MB, Thunnissen FB, Brambilla E, Hasleton P, Steele R, Hammar SP, et al. Pulmonary atypical carcinoid: predictors of survival in 106 cases. Hum pathol. 2000;31(10):1255-65.
- Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The Pathologic Classification of Neuroendocrine Tumors: A Review of Nomenclature, Grading, and Staging Systems. Pancreas. 2010;39(6):707-12.
- Rekhtman N. Neuroendocrine tumors of the lung: An update. Arch Path Lab Med. 2010;134(11):1628-38.
- Williams ED, Sandler M. The classification of carcinoid tumours. The Lancet. 1963;281(7275):238-9.
- Hubalewska-Dydejczyk A, Fröss-Baron K, Gołkowski F, Sowa-Staszczak A, Mikołajczak R, Huszno B. 99m Tc-EDDA / HYNIC-Octreotate in Detection of Atypical Bronchial Carcinoid. Exp Clin Endocrinol Diabetes. 2007;115(1):47-9.
- Morandi U, Casali C, Rossi G. Bronchial typical carcinoid tumors. In Seminars in thoracic and cardiovascular surgery. 2006;(18)3:191-8.
- Moertel CG. Karnofsky memorial lecture. An odyssey in the land of small tumors. J Clin Oncol. 1987;5 (10):1502-22.
- Kwekkeboom DJ, Lamberts SWJ, Habbema JDF, Krenning EP. Cost Effectiveness Analysis of Somatostatin Receptor Scintigraphy. J Nucl Med. 1996;37(6):886-92.
- Kong G, Johnston V, Ramdave S, Lau E, Rischin D, Hicks RJ. HighAdministered Activity In-111 Octreotide Therapy with Concomitant Radiosensitizing 5FU Chemotherapy for Treatment of Neuroendocrine Tumors: Preliminary Experience. Cancer Biother Radiopharm. 2009;24(5):527-33.
- Binderup T, Knigge U, Loft A, Mortensen J, Pfeifer A, Federspiel B, et al. Functional imaging of neuroendocrine tumors: a head-tohead comparison of somatostatin receptor scintigraphy, 123I-MIBG scintigraphy, and 18F-FDG PET. J Nucl Med. 2010;51(5):704-12.
- Blechacz B, Gores GJ. Positron emission tomography scan for a hepatic mass. Hepatology. 2010;52 (6):2186-91.
- Öberg KE. Management of neuroendocrine tumors: current and future therapies. Expert Rev Endocrinol. Metab. 2011;6 (1):49-62.
- Pinsky PF, Church TR, Izmirlian G, Kramer BS. The National Lung Screening Trial: results stratified by demographics, smoking history, and lung cancer histology. Cancer. 2013;119(22):3976-83.