Mini Review
Tarpgaard et al is: Repurposing of Drugs in Chemotherapy Resistant Colorectal Cancer
Line Tarpgaard1, SofieOtzen Bagger2, Per Pfeiffer1, Nils Brünner2 and Jan Stenvang2*
1Department of Oncology, Odense University Hospital, Denmark
2Department of Drug Design and Pharmacology, Faculty of Health and Medical Sciences, University of Copenhagen, Denmark
*Corresponding author: Jan Stenvang, Department of Drug Design and Pharmacology, Faculty of Health and Medical Sciences, University of Copenhagen, Denmark
Published: 11 Dec, 2017
Cite this article as: Tarpgaard L, Bagger SO, Pfeiffer P,
Brünner N, Stenvang J. Tarpgaard
et al is: Repurposing of Drugs in
Chemotherapy Resistant Colorectal
Cancer. Clin Oncol. 2017; 2: 1382.
Abstract
Chemotherapy resistance remains a major clinical problem in the current treatment of cancer patients and chemotherapy resistance almost inevitably occursin metastatic cancer patients.In colorectal cancer patients, the response rates to first line chemotherapy therapy is approximately 50%, dropping to approximately 10-20% in second line treatment and no standard third line treatment exists. Thus, there is an obvious and acute need for novel drugs to increase response rates. Here, we focus on the promising idea of repurposing existing drugs in clinical trials with chemotherapy resistant colorectal cancer patients and suggest a general clinical trial design that could be employed to test repurposed drugs.
Introduction
Drug resistance,being de novo or acquired, represents a major clinical challenge in today’s
treatment of cancer patientsresulting in a large fraction of patients receiving chemotherapy without
any beneficial effects while they almost all experience drug related site effects [1]. Overall, it has been
estimated that the response rates of cancer patients to chemotherapy are as low as 25% [2]. One way
to tackle this problem is to develop and use predictive biomarkers, which can be used to allocate the
right treatment to the right patient at the right time. Predictive biomarkers can be markers for drug
sensitivity or markers for drug resistance [3]. Predictive biomarkers appear to have most impact
in early line treatment since many patients will develop cross resistance and thereby multi drug
resistance over the course of different treatment lines. In fact, drug resistance may cause treatment
failure in over 90% of patients with metastatic cancer [4]. This short commentary will focus on the
use of repurposing drugs to overcome drug resistance in colorectal cancer (CRC) patients as drug
resistance is considered the single most important obstacle to greater success with chemotherapy
for patients suffering with this disease [5].
CRC still represents a major health problem being the third most frequent cancer form in males
and second most frequent in females with 1.4 million new cases and 700,000 deaths from CRC in
2012 in the Western World [6]. These numbers clearly illustrate that treatment of CRC is still far
from optimal and despite the recent introduction of a number of new treatment options for this
disease, a large number of patients continue to die from CRC.
Surgery is still the only effective treatment of CRC. However, the success of the surgical
treatment entirely depends on the stage of disease being presented at time of surgery. Patients with
high risk stage II or stage III CRC are offered adjuvant treatment in order to reduce the risk of
disease recurrence after surgical removal of all visible lesions. Adjuvant medical treatment of CRC
has not changed significantly for a decade and approximately 25% of the treated stage II patients and
40% stage III patients will experience disease recurrence despite systemic adjuvant treatment [7]. In
patients with metastatic CRC (mCRC) today’s treatment includes conventional chemotherapy based
on only three cytotoxic drugs: fluorouracil (5-FU), oxaliplatin and irinotecan. These drugs are used
in combinations of 5-FU/capecitabine/S1 plus leucovorinin combination with either oxaliplatin
or irinotecan, and in some cases in combination with targeted biological treatment (EGF-receptor
blockade or anti-angiogenic treatment) [8-10]. The response rate to first line treatment of mCRC
is approximately 50% independent on which of the above mentioned drug combinations that are
used but unfortunately, most of these patients will develop drug resistant disease as evidenced by
progression of their cancer [11]. Objective response rates to second line treatment are reduced
to 10-20% depending on the drug combination used [11]. However, all of these patients will
experience disease recurrence/progression. There is no standard third
line therapy, but the third line treatment chosen by the oncologist
can induce a short increase in progression-free survival and overall
survival but without meaningful tumor regression.
From the above mentioned it is evident that drug resistance in CRC
represents one of the most important clinical challenges in today’s
treatment of CRC patients. This problem can be alleviated in several
ways, including improved early diagnosis (down-staging), reliable
predictive biomarkers, novel drugs/drug combinations, repurposing
of existing drugs, and optimized administration schedules.
We have chosen to focus on the very promising possibility
to repurpose/reposition existing drugs. Repurposing of drugs is
an alternative to the classical pipeline of drug development and
there has been increasing interest in analyzing anti-cancer activity
of non-cancer drugs already in the existing FDA approved drug
armamentarium [12]. Overall, the repurposing strategy possesses two
major advantages. Firstly, prior knowledge of e.g. pharmacokinetics,
toxicities and dosing can be utilized in the drug repurposing.
Secondly, many of the candidates for anti-cancer drug repurposing
are at very low cost in contrast to the very expensive novel agents.
Obviously, the candidate drugs for repurposing in CRC need to
be tested in a suitable clinical set-up. Currently, we are conducting a
“windows of opportunity” Phase II clinical trial in Denmark in which
we repurpose an anti-cancer agent (epirubicin) in mCRC [13]. This
is a biomarker (Topoisomerase IIa gene copy number) guided trial
based on Simon’s two stages Mini-max design, which ensures early
study termination if there is insufficient effect [14].
When repurposing a drug, which is not currently used as anticancer
agent, there is a potential risk that the drug in combination
with the standard chemotherapy hasunexpected toxic effects. Thus,
we suggest a “three + three dose escalating” clinical trial design in
which a run-in study is conducted to minimize the risk of adverse
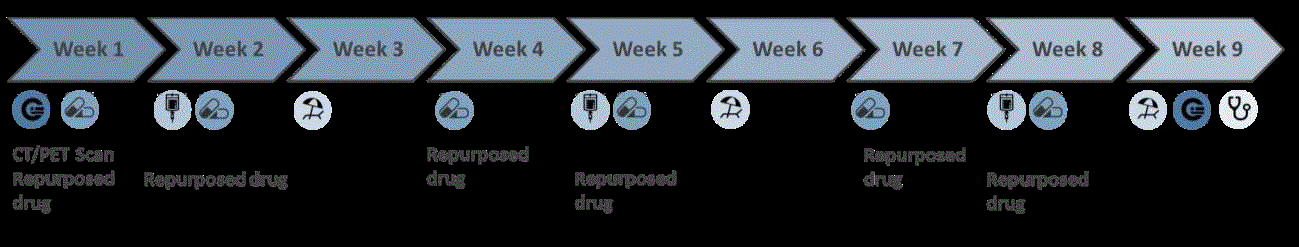
toxic events 8 (Figure 1). The concept is that patients are included
in cohorts of three at progressively higher dose levels of the
repurposed drug. All patients will receive standard concentrations
of chemotherapy in combination with the repurposed drug. Initially,
three eligible patients are included with standard chemotherapy
in combination with the repurposed drug in a dose that is 50% of
the previously established maximum tolerated dose (MTD). If dose
limiting toxicity (DLT) is observed in one of three patients during
the first course of treatment, three additional patients will be added
at the respective dose level. Dose escalation will continue only if none
of three or one of six patients experience DLT. In this case, dose
escalation will continue to 75%of the MTD and the same procedure
will be followed. At the highest dose of the repurposed drug six
patients will be included. These six patients will also be included in
the following Phase II study to minimize the number of patients in
the trial. The phase II study will follow the Simon’s two stages Minimax
design [14].
We are currently planning such a repurposing study with
disulfiram (Antabuse) in patients with mCRC who failed first line
systemic treatment. In preclinical models (isogenic pairs of parental
chemotherapy sensitive and chemotherapy resistant cell lines) we have
observed that disulfiram in combination with copper significantly
increases the anti-tumor effects of irinotecan in oxaliplatin resistant
CRC cells or increases the effects of oxaliplatin in irinotecan resistant
mCRC cells [15]. The study will also be a “window of opportunity”
study with a run-in study preceding the main phase II study as
described above. We consider this design to be a general design for
clinical trials of repurposed drugs when given in combination with
standard chemotherapy in patients with metastatic cancer.
Figure 1
Figure 1
Design for clinical Phase II studies with repurposed drugs in combination with chemotherapy in cancer patients that have become resistant to the
chemotherapy.
References
- Fojo T, Parkinson DR. Biologically targeted cancer therapy and marginal benefits: are we making too much of too little or are we achieving too little by giving too much? Clin Cancer Res 2010; 16(24): 5972-80.
- Spear BB, Heath-Chiozzi M, Huff J. Clinical application of pharmacogenetics. Trends Mol Med 2001; 7(5): 201-4.
- Jorgensen JT. Clinical application of companion diagnostics. Trends Mol Med. 2015; 21(7): 405-7.
- Longley DB, Allen WL, Johnston PG. Drug resistance, predictive markers and pharmacogenomics in colorectal cancer. Biochim Biophys Acta. 2006; 1766(2): 184-96.
- Hammond WA, Swaika A, Mody K. Pharmacologic resistance in colorectal cancer: a review. Ther Adv Med Oncol 2016; 8: 57-84.
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65(2): 87-108.
- Andre T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009; 27(19): 3109-16.
- Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010; 28(31): 4697-705.
- Tveit KM, Guren T, Glimelius B, Pfeiffer P, Sorbye H, Pyrhonen S, et al. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: the NORDIC-VII study. J Clin Oncol 2012; 30(15): 1755-62.
- Van CE, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009; 360(4): 1408-17.
- Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 2004; 22: 229-37.
- Pantziarka P, Bouche G, Meheus L, Sukhatme V, Sukhatme VP, Vikas P. The Repurposing Drugs in Oncology (ReDO) Project. Ecancermedicalscience 2014; 8: 442.
- Tarpgaard LS, Qvortrup C, Nygard SB, Nielsen SL, Andersen DR, Jensen NF, et al. A phase II study of Epirubicin in oxaliplatin-resistant patients with metastatic colorectal cancer and TOP2A gene amplification. BMC Cancer 2015; 16: 91.
- Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials 1989; 10: 1-10.
- Stenvang J, Keinicke H, Nielsen SL, Jandu H, Bartek J, Brünner N. Repurposing disulfiram as a potential novel treatment of drug-resistant metastatic colorectal cancer. In Proceedings of the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics: Discovery, Biology, and Clinical Applications, Philadelphia, PA, USA. 2017.