Case Report
Simultaneous Tubulovillous Adenoma with Focal Adenocarcinoma and Neuroendocrine Tumor in Ampulla of Vater: Report of One Case
Yueh-Tsung Lee*
Department of Surgery, Chang-Bing Show Chwan Memorial Hospital, Taiwan
*Corresponding author: Yueh-Tsung Lee, Department of Surgery, Chang-Bing Show Chwan Memorial Hospital, Taiwan
Published: 08 Dec, 2017
Cite this article as: Lee Y-T. Simultaneous Tubulovillous
Adenoma with Focal Adenocarcinoma
and Neuroendocrine Tumor in Ampulla
of Vater: Report of One Case. Clin
Oncol. 2017; 2: 1380.
Case Report
A 56 year-old male with medical control for diabetes for years, was referred to our hospital
due to abdominal discomfort accompanied with body weight loss 10 kg within 6 months. The
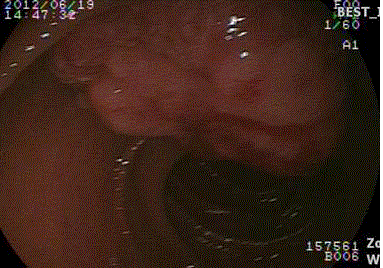
gastroscopy demonstrated a tumor in Ampulla of Vater (Figure 1). The abdominal CT and MRI
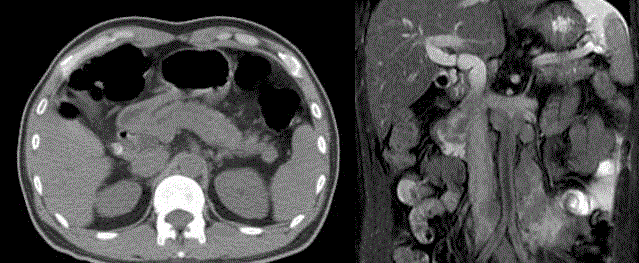
revealed a tumor in duodenum with dilatation of common bile duct and pancreatic duct dilatation,
and para-aortic lymph nodes metastases (Figure 2). The patient underwent pylorus-preserving
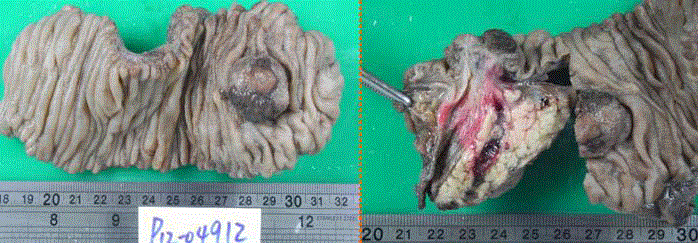
pancreaticduodenectomy (PPPD) and para-aotirc lymph nodes sampling (Figure 3). Pathologically,
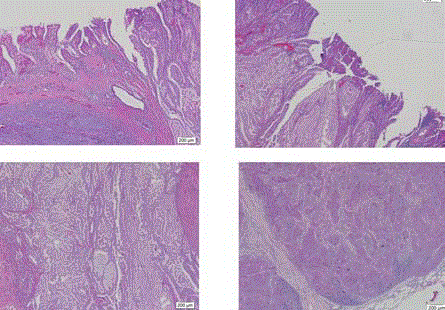
the tumor was consisted of tubulovillous adenoma, adenocarcinoma and neuroendocrine tumor
(NET) (mitosis: 10/10HPF, Ki-67: 5%). The sampled lymph nodes revealed metastasis from NET
(Figure 4). The neuroendocrine tumor presented with expression of
chromogranin (Figure 5). The patient received cytotoxic chemotherapy
but developed liver metastases 9 month after the surgery (Figure 6).
The PET scan revealed hyper-metabolic lesions in liver and paraaortic
regions (Figure 7). The In-111-DTPA-Octreotide scan showed
lesions with abnormal accumulation of radiotracer in segment 8 of
liver, peri-caval areas, para-aortic and common iliac areas (Figure
8). We prescribed transarterial chemoembolization (TACE) for the
liver tumors and somatostatin analogs for systemic treatment (Figure
9). The patient took multiple kinase inhibitors (sunitinib malate)
and cytotoxic chemotherapy (Darcarbazine) for refractory tumors.
However, the patient died of disease progression two years and six
months after surgery.
The carcinoid tumor was replaced by neuroendocrine tumors
(NETs) in nomenclature recently [1,2]. The incidence of NETs is
increasing in the past decades [3]. The NETs are classified according
to the mitosis and Ki-67 proliferation factor which correlate with
prognosis [4]. The gastroenteropancreatic neuroendocrine tumors
(GEP-NETs) have different frequency in different organs with
differently biological behavior [2]. The survival time is poor while the
disease presents as a metastatic disease with average of 33 months
[3]. Mixed adenoneuroendocrine carcinoma (MANEC) cases
arising from different organs were reported [5-7]. However, our
present case was not MANEC because of separate tumor content in
a mass. Besides surgery and chemotherapy, target therapy such as
somatostatin analogs is effective in tumor with expression of radiotracer
acumination [8]. For our case, para-aortic lymph nodes were
sampled during laparotomy and the frozen section pathologic reports
revealed suspicion of carcinoid instead of carcinoma. Therefore,
we finished PPPD and waited for analysis for Ki-67 and mitosis to
classify the tumor grading. To our beast knowledge, our present case
has never been reported.
Figure 1
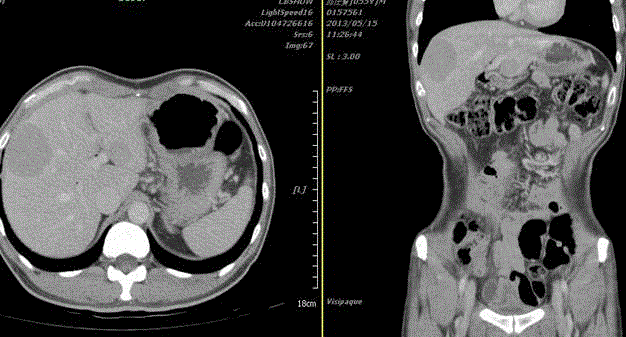
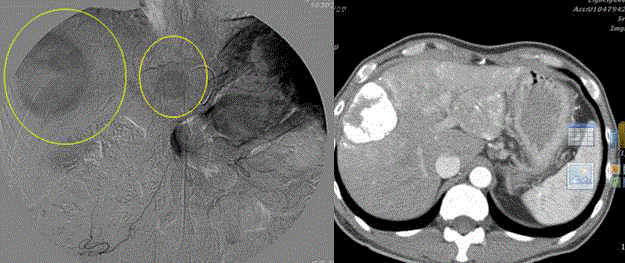
Figure 2
Figure 2
The abdominal CT revealed a protruding mass from CBD and pancreatic duct junction (left). The
abdominal MRI showed the duodenal tumor arising from Ampulla of Vaer and several lymph nodes enlargement
in paraaortic region (right).
Figure 3
Figure 3
The specimens revealed a 3-cm of tumor arising from Ampulla of Vater (left) and the dilated CBD and
pancreatic duct (right).
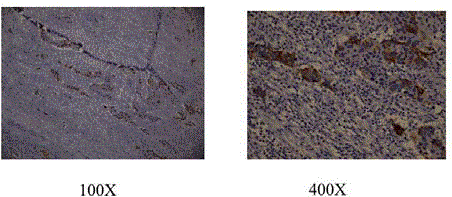
Figure 4
Figure 4
The pathologic reports revealed the tumor consisted of tubulovillous
adenoma (superior, left) with adenocarcinoma in the tumor margin (superior,
right) and neuroendocrine tumor (inferior, left). The metastatic lymph nodes
originated from neuroendocrine tumor (inferior, right) (40X).
Figure 5
Figure 5
The tumor cells express specific staining by chromograinin
antibodies immunohistochemically.
Figure 6
Figure 6
The abdominal CT revealed two isodense tumors in liver (S3,S8)
and multiple paraaortic lymph adenopathy (not shown).
Figure 7
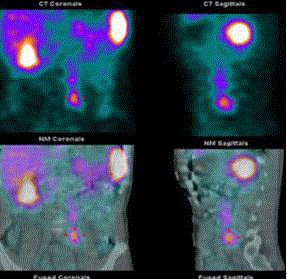
Figure 8
Figure 8
In-111 octreotide scan revealed abnormal tracer acumination in the
liver (S8), peri-caval and paraaortic regions.
Figure 9
Figure 9
The transarterial chemo-emboliztion (TACE) was done (left) and
follow-up abdominal CT revealed hyperdesne mass after TACE (right).
References
- Sundin A, Vullierme MP, Kaltsas G, Plockinger U. Mallorca Consensus Conference p, European Neuroendocrine Tumor S: ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: radiological examinations. Neuroendocrinology. 2009; 90(2): 167-183.
- Niederle MB, Hackl M, Kaserer K, Niederle B. Gastroenteropancreatic neuroendocrine tumours: the current incidence and staging based on the WHO and European Neuroendocrine Tumour Society classification: an analysis based on prospectively collected parameters. Endocrine-related cancer. 2010; 17(4): 909-918.
- Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. Journal of clinical oncology: j the American Society of Clin Onc. 2008; 26(18): 3063-3072.
- Plockinger U, Wiedenmann B, de Herder WW. ENETS Consensus Guidelines for the Standard of Care in Neuroendocrine Tumors. Neuroendocrinology. 2009; 90(2): 159-161.
- August C, Maker AV, Weisenberg E. Simultaneous Occurrence of Glandular and Neuroendocrine Components in Lymph Node Metastasis of Gastric MANEC. Int j surg path. 2015; 23(5): 375-376.
- Veits L, Lang-Schwarz C, Volkholz H, Falkeis C, Vieth M, Schulz H. Mixed adenoneuroendocrine carcinoma (MANEC) of the esophagogastric junction predominantly consisting of poorly differentiated neuroendocrine carcinoma. Endoscopy 2013.
- Paniz Mondolfi AE, Slova D, Fan W, Attiyeh FF, Afthinos J, Reidy J. Mixed adenoneuroendocrine carcinoma (MANEC) of the gallbladder: a possible stem cell tumor? Pathology international 2011; 61(10): 608-614.
- Kwekkeboom DJ, Krenning EP, Lebtahi R, Komminoth P, Kos-Kudla B, de Herder WW. Plockinger U, Mallorca Consensus Conference p, European Neuroendocrine Tumor S: ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: peptide receptor radionuclide therapy with radiolabeled somatostatin analogs. Neuroendocrinology 2009; 90(2): 220-226.