Review Article
Molecular Hydrogen as a Novel Therapeutic Tool in Situations of Increased Production of Free Radicals
Ján Slezák1*, Juraj Surový2, Jozef Buday2, Branislav Kura1
1Institute for Heart Research, Slovak Academy of Sciences, Dúbravskácesta, Bratislava, Slovakia
2Electrotechnical Research and Projecting Company, Trenčianska, Dubnica, Slovakia
*Corresponding author: Ján Slezák, Institute for Heart Research, Slovak Academy of Sciences, Slovakia
Published: 26 Nov, 2017
Cite this article as: Slezák J, Surový J, Buday J, Kura
B. Molecular Hydrogen as a Novel
Therapeutic Tool in Situations of
Increased Production of Free Radicals.
Clin Oncol. 2017; 2: 1367.
Abstract
Excessive production of oxygen and nitrogen radicals has been regarded as a causative common
denominator of many pathological processes. The increase in the rate of radiation-induced heart
disease has emphasized the need to seek for new therapeutic targets to mitigate the negative impact
of radiation on the heart.
Scavenging of free radicals can act preventively or therapeutically. A number of substances that
preferentially react with free radicals can serve as scavengers, thus increasing internal capacity/
activity of endogenous antioxidants.
Our results demonstrate that in the hearts of rats injured by irradiation H2 administration led to a
significant decrease in MDA and TNF-α levels as well as to miRNAs return almost to control levels.
Molecular hydrogen (H2) reacts with strong oxidants, such as hydroxyl and nitrosyl radicals in cells,
allowing to use its potential for preventive and therapeutic applications in situations with excessive
free radicals formation. H2 rapidly diffuses into tissues and cells without affecting signaling reactive
species. H2 also reduces oxidative stress by regulating gene expression and functions as an antiinflammatory
and anti-apoptotic agent.
Keywords: Molecular hydrogen; miRNA; ROS; Radiation; Malondialdehyde; Inflammation
Introduction
There are numerous situations with established excessive production of ROS, like radiation,
ischemia and reperfusion, inflammation, rheumatoid arthritis, cancer, diabetes, and number of
other pathologies including aging [1].
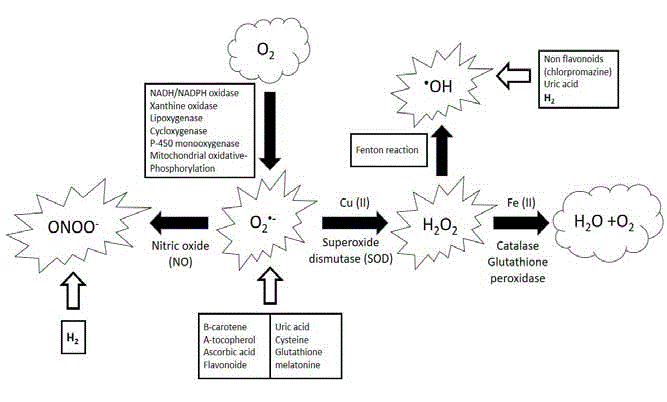
Oxidative stress occurs due to the imbalance between the production of reactive oxygen and
nitrogen species and the capability of innate biological systems to eliminate reactive intermediates
(Figure. 1).
Unlike superoxide, which can be detoxified by superoxide dismutase, the hydroxyl radical
cannot be eliminated by an enzymatic reaction. Mechanisms of scavenging peroxylradicals to
protect cellular structures includes endogenous antioxidants such as melatonin and glutathione,
and dietary antioxidants such as mannitol and vitamin E.
Formation of peroxynitritein vivo has been ascribed to the reaction of the free radical superoxide
with the free radical - nitric oxide as follows:
•O−2 + •NO → ONO−2 and H2O2 + NO−2 → ONOO− + H2O.
In vivo, peroxynitrite generation represents a crucial pathogenic mechanism in conditions such
as stroke, myocardial infarction and chronic heart failure.
Oxidative stress and/or nitrosative stress caused by H2O2 and •NO (resulting in peroxynitrite
formation) induces enzymes involved in anti oxidation and oxidative damage [2,3]. Hydroxyl and
nitrosyl radicals represent the major cause of the destruction of biomolecules either by a direct
reaction or by triggering a chain reaction of free radicals.
Scavenging of free radicals acts preventively or therapeutically. A number of substances serve as
scavengers, protecting cells and tissues against oxidative damage. Early research identified sulfurcontaining
antioxidants, later on, low molecular weight antioxidants, ascorbic acid, tocopherols,
polyphenols, and thiols such as glutathione were used. Recently, gene therapy-mediated over
expression of the SOD using recombinant adeno-associated viral
vectors and administration of manganese superoxide dismutaseplasmid
liposomes (MnSOD-PL) are studied [4].
To attenuate the harmful effects of oxidative stress and ROS, one
can eliminate them by medicinal gases (such as carbon monoxide
(CO), hydrogen sulphide (H2S), and molecular hydrogen (H2), or
induce ROS-resistant proteins and antioxidant enzymes to antagonize
oxidative stress [5].
Many attempts have been made to inhibit ROS production and
to limit the extent of reperfusion injury. However, the administration
of ROS scavengers at the time of reperfusion has brought conflicting
results that can be partially explained by the dual role of ROS in I/R
[6], since ROS play an important role as signaling molecules as well.
Molecular Hydrogen (H2)
Therapeutic effects of molecular hydrogen for a wide range of
disease models and human diseases have been investigated thoroughly
since 2007 and the positive effects were reported in essentially all
organs covering many disease categories and disease models [7,8].
One of the main effects of H2 is hydroxyl radicals scavenging by
reaction: H2 + ·OH = H2O + H· and followed by H· + O2
- = HO2-reaction [9,10]. This way H2 is able to provide cytoprotection by
increasing other antioxidant enzymes such as superoxide dismutase
and catalase [11].
H2 is superior to some antioxidants since it can selectively
scavenge the deleterious hydroxyl radical while preserving other
important reactive oxygen and nitrogen species for signaling.
Emerging evidence has demonstrated the pleiotropic therapeutic
effects of molecular hydrogen in a variety of animal disease models as
well as in many human diseases [12,13,14,15].
Ways of H2 applications
Hydrogen can be applied in form of hydrogen-rich water,
hydrogen gas, by administration of hydrogen-rich saline and/or by
dropping H2-saline into the eyes. Hydrogen water is mostly given ad
libitum. Hydrogen gas of less than 4 % is given by inhalation.
Hydrogen-rich water (prepared by different procedures like
dissolving molecular hydrogen into water under high pressure or
dissolving hydrogen generating tablets in water) generally shows a
more prominent effect than hydrogen gas, although the amount of
hydrogen taken up by hydrogen water is ~100 times less than that
given by hydrogen gas [16].
H2 reduces oxidative stress not only by direct reactions, with
strong oxidants, but also indirectly by regulating various gene
expressions and modulating cell signaling [7,8,17].
Effects of hydrogen on various diseases can be summarized and
have been attributed to four major molecular mechanisms:
1 - specific scavenging activity of hydroxyl radicals
2 - scavenging activity of peroxynitrite
3 - alterations of gene expressions
4 - signal modulating activities.
Figure 1
Figure 1
Production of superoxide, peroxynitrite and hydroxyl radicals and some of specific scavangers in vivo.
Methods
As a source of free radicals, we used radiation that interacts with
the water present in the cells, resulting in its hydrolysis and formation
of free radicals (especially hydroxyl OH).
Wistar rats at the age of 12 weeks, weighing approximately 250
g, were irradiated with a single dose of 25 Gy (6-7 Gy / min) and/or
10 Gy (4-5 Gy / min.) focused on the mediastinal area. Therapeutic
application of molecular hydrogen has been performed by different
delivery methods including inhalation, drinking hydrogen-rich
water prepared by dissolving molecular hydrogen into water under
high pressure and/or infusion of hydrogen-saturated solutions.
Concentrations of malondialdehyde (MDA) in rat serum were
determined by spectro photometric method using a commercially
available TBARS assay kit (Cayman Chemical, Michigan, USA).
TNF-α expression was analyzed from the left ventricle tissue using the
Western blot method. The effect of molecular hydrogen in irradiated
rat myocardium based on the changes of expression of miRNA 1, 15b
and 21 levels were measured by RT-qPCR.
Results
The results of our experiments in irradiated rats demonstrated that H2 was followed by a decrease in MDA and TNF-α levels as well as miRNAs return almost to control levels.
Discussion
Possible molecular mechanisms of H2 action
Although the underlying mechanisms were initially proposed
as selective extinctions of hydroxyl radical and peroxynitrite,
the signaling pathway regulation effect of molecular hydrogen
by modulating a various molecules expressions/activities, gene
expression and microRNA may also account for the ultimate effects
on ischemia-reperfusion injury, infammation, apoptosis, metabolic
disorders, allergy, radiation injury, dementia as well as aging [8,17-
22].
H2 was shown to exhibit multiple functions including antiinflammatory,
anti-apoptotic, anti-allergic, and antioxidant
properties, as well as regulation of cell differentiation and involvement
in energy metabolism. Molecular hydrogen modulates various signal
transduction pathways and the expression of many genes [14,23] and
regulates more components of MAPK signal transduction pathways
[24]. It was shown that H2 modulates Ca2+ signal transduction and
regulates gene expression [15].
As demonstrated in our experiments, molecular hydrogen
modulates in irradiated rats miRNA expression of miR-1, miR-15b
and miR-21 [17] and returns the values of these miRNAS to control
levels.
As shown in our experiments, H2 can protect myocardium
from radiation-induced injury and decrease lipid peroxidation of
myocardium demonstrated by decrease of malondialdehyde (MDA)
levels, and increase myocardial endogenous antioxidants in vivo.
Results are in consent with work of [25] who have investigated the
cardio protective properties of molecular hydrogen by pre-treating
mice with hydrogen-rich water prior to irradiation where 90% of the
mice without hydrogen-rich water pretreatment died, while 80% of
the mice with hydrogen survived. Hydrogen pre-treatment proved
to exert cardio protective properties by decreasing malondialdehyde
(MDA) and eight-hydroxydeoxyguanosine (8-OHdG) levels as
opposed to the non-treatment counterparts, which showed increased
levels of those oxidative stress markers.
It was shown that ONOO-has the potential to regulate
gene expressions through the nitration of factors involved in
transcriptional regulation [26]. Drinking hydrogen water suppresses
the nitration of proteins; thus, it is possible that small amount of H2
consumed by drinking hydrogen-rich water influences nitration in
in vivo experiments and results in regulatory as well as anti-oxidative
effects. These results also agree with the finding of Cardinal et al. that
H2 suppressed the nitration of proteins [27].
Hydrogen was shown to act also by inhibiting LPS/IFN γ-induced
nitric oxide production in macrophages, resulting in decreased
inflammation. Hydrogen was able to modulate signal transduction,
which suggests that hydrogen is a signal modulator [28]. Shi has
proposed that molecular hydrogen may be able to affect signal
transduction by interacting with metallo proteins, since metal ions
can be a possible binding site for hydrogen [29]. H2 can reveal
cytoprotection by preventing the activation of caspase-3 and reduce
apoptosis [30].
As shown in our experiments, molecular hydrogen is able to
inhibit inflammation caused by irradiation and the TNF-α/NF-κB
pathway [31] as well as the Ras-ERK1/2-MEK1/2 and PI3K/Akt
pathways [32,33]. Huang demonstrated that NF-κB is activated by
hydrogen administration and this can be correlated with the elevated
levels of the anti apoptotic protein Bcl-2. The protective effects of
hydrogen was reversed by a chemical inhibitor of NF-κ B [34].In
consent with this study is a work of showing that treatment with
hydrogen rich saline restored expression of the anti-apoptotic factor,
Bcl-2, and decreased protein expression of Bax, a pro-apoptotic factor
[35].
A study performed by Sun, demonstrated that hydrogen-rich
saline was able to be effective against myocardial ischemia and
reperfusion injury in rats. The findings of the study were that there
were significant decreases in infarct sizes, MDA concentrations and
8-OHdG levels in at risk areas, as well as evidence that hydrogen was
able to inhibit the caspase-3, and attenuate apoptosis [30].
Zalesak published results of myocardial ischemia/reperfusion
experiment where H2 saturated Krebs–Henseleit solution was
administered in a setting of myocardial hypoxic post conditioning
and significantly decreased infarct size. Application of hydrogen
appears to be beneficial in a setting of hypoxic post conditioning and
to facilitate its cardio protective efficiency [22].
As demonstrated in our experiments, in irradiated rats, molecular
hydrogen modulates miRNA expression of miR-1, miR-15b and miR-
21 [36] and miR-9, miR-21, and miR-199 [37,38], and possibly many
others. Analysis of miRNA profiles of hippocampal neurons during
I/R injury revealed that hydrogen inhibits I/R-induced expression
of the miR-200 family by reducing ROS production, which has led
to suppression of cell death [38]. However, modulation of miRNA
expression cannot solely explain all the biological effects mediated by
hydrogen. Mechanisms underlying modulated miRNA expression
remain to be elucidated.
miRNAs show different expression patterns in the normal and
diseased heart and some data indicate that miRNA expression may
represent an efficient diagnostic marker of heart disease [39]. Ikeda
showed that the specific class of heart disease can be predicted
using miRNAs expression with the probability of 69% [40]. As a
great advantage for the use of miRNAs as biomarkers or potential
therapeutic targets is its stability under many conditions [41].
In this regard microRNAs (miRNAs) have received considerable
interest. MiRNAs regulate post-transcriptional gene expression
by their ability to target various mRNA sequences because of their
imperfect pairing with mRNAs. It has been recognized that miRNAs
modulate a diverse spectrum of cardiac function with developmental,
patho physiological, and clinical implications. This makes them
promising potential targets for diagnosis and treatment [17].
Conclusion
Targeted application of molecular hydrogen (H2>) as one of the
potent scavengers may be used in the prevention and treatment of
ROS-related diseases.H2 reacts with strong oxidants, such as hydroxyl
and/or nitrosyl radicals that makes it possible to utilize its potential
for preventive and therapeutic applications. H2 is able to reduce
oxidative stress also by regulating gene expression, and acts as an
anti-inflammatory and anti-apoptotic agent.
miRNAs represent potential targets for modulation and
manipulation to be used as biomarkers and therapeutic instruments.
Application of molecular hydrogen in situations with excessive
production of free radicals and, in particular, hydroxyl and
nitrosyl radicals increases internal capacity/activity of endogenous
antioxidants. It is simple and efficient method that deserves special
attention.
H2 may be novel effective clinical tool for treating oxidative
stress-related diseases.
Acknowledgement
This study was supported by grants APVV-0241-11, VEGA 2/0021/15 and partially NIH R37 HL051045, VEGA SR 2/0201/15, APVV-0102-11 and APVV-15-0376.
References
- Majzunova M, Dovinova I, Barancik M, Chan JY. Redox signaling in pathophysiology of hypertension. J Biomed Sci. 2013; 20: 69.
- Endo J, Sano M, Katayama T, Hishiki T, Shinmura K, Morizane S, et al. Metabolic remodeling induced by mitochondrial aldehyde stress stimulates tolerance to oxidative stress in the heart. Cir. Res. 2009; 105(11): 1118-27.
- Jazwa A, Cuadrado A. Targeting heme oxygenase-1 for neuroprotection and neuroinflammation in neurodegenerative diseases. Curr Drug Targets. 2010; 11(12): 1517-31.
- Citrin D, Cotrim AP, Hyodo F, Baum BJ, Krishna MC, Mitchell JB. Radioprotectors and mitigators of radiation-induced normal tissue injury. Oncologist. 2010; 15(4): 360-71.
- Noda M, Fujita K, Lee CH, Yoshioka T. The principle and the potential approach to ROS-dependent cytotoxicity by non-pharmaceutical therapies: optimal use of medical gases with antioxidant properties. Curr Pharm Des. 2011; 17(22): 2253-63.
- Flaherty JT, Pitt B, Gruber JW, Heuser RR, Rothbaum DA, Burwell LR, et al. Recombinant human superoxide dismutase (h-SOD) fails to improve recovery of ventricular function in patients undergoing coronary angioplasty for acute myocardial infarction. Circulation 1994; 89(5): 1982-91.
- Ohta S. Molecular hydrogen as a preventive and therapeutic medical gas: initiation, development and potential of hydrogen medicine. PharmacolTher. 2014; 144(1): 1-11.
- Slezák J, Kura B, Frimmel K., Zálešák M, Ravingerová T, Viczenczová C, et al. Preventive and therapeutic application of molecular hydrogen in situations with excessive production of free radicals. Phys Res. 2016; 65(1): S11-28.
- Dole M, Wilson FR, Fife WP. Hyperbaric hydrogen therapy: a possible treatment for cancer. Science. 1975; 190(4210): 152-4.
- Gharib B, Hanna S, Abdallahi OM, Lepidi H, Gardette B, De Reggi M. Anti-inflammatory properties of molecular hydrogen: investigation on parasite-induced liver inflammation. C R AcadSci III. 2001; 324(8): 719-24.
- Xie K, Yu Y, Pei Y, Hou L, Chen S, Xiong L, et al. Protective effects of hydrogen gas on murine polymicrobial sepsis via reducing oxidative stress and HMGB1 release. Shock. 2010; 34(1): 90-7.
- Iketani M, Ohsawa I. Molecular hydrogen as a neuroprotective agent. CurrNeuropharmacol. 2016; 15(2): 324-31.
- Huang CS, Kawamura T, Toyoda Y, Nakao A. Recent advances in hydrogen research as a therapeutic medical gas. Free Radic Res. 2010; 44(9): 971–82.
- Ohta S. Recent progress toward hydrogen medicine: potential of molecular hydrogen for preventive and therapeutic applications. Curr Pharm Des. 2011; 17(22): 2241-52.
- Dixon BJ, Tang J, Zhang JH. The evolution of molecular hydrogen: a noteworthy potential therapy with clinical significance. Med Gas Res. 2013; 3(1): 10.
- Ito M, Hirayama M, Yamai K, Goto S, Ito M, Ichihara M, et al. Drinking hydrogen water and intermittent hydrogen gas exposure, but not lactulose or continuous hydrogen gas exposure, prevent 6-hydorxydopamine-induced Parkinson's disease in rats. Med Gas Res. 2012; 2(1): 15.
- Kura B, Babal P, Slezak J. Implication of microRNAs in the development and potential treatment of radiation- induced heart disease. Can J PhysiolPharmacol. 2017; 95(10): 1236-44
- Ichihara M, Sobue S, Ito M, Ito M, Hirayama M, Ohno K. Beneficial biological effects and the underlying mechanisms of molecular hydrogen-comprehensive review of 321 original articles. Med Gas Res. 2015; 5: 12.
- Hara F, Tatebe J, Watanabe I, Yamazaki J, Ikeda T, Morita T. Molecular hydrogen alleviates cellular senescence in endothelial cells. Circ J. 2016; 80(9): 2037-46.
- Li Q, Yu P, Zeng Q, Luo B, Cai S, Hui K, et al. Neuroprotective effect of hydrogen-rich saline in global cerebral ischemia/reperfusion rats: up-regulated tregs and down-regulated miR-21, miR-210 and NF-kappaB expression. Neurochem Res. 2016; 41(10): 2655-65.
- Shao A, Wu H, Hong Y, Tu S, Sun X, Wu Q, et al. Hydrogen-rich saline attenuated subarachnoid hemorrhage-induced early brain injury in rats by suppressing inflammatory response: possible involvement of NF-kappaB pathway and NLRP3 inflammasome. MolNeurobiol. 2016; 53(5): 3462-76.
- Zálešák M, Kura B, Graban J, Farkašová V, Slezák J, Ravingerová T. Molecular hydrogen potentiates beneficial anti-infarct effect of hypoxic postconditioning in isolated rat hearts: a novel cardioprotective intervention. Can J PhysiolPharmacol. 2017; 95(8): 888-93.
- Hanaoka T, Kamimura N, Yokota T, Takai S, Ohta S. Molecular hydrogen protects chondrocytes from oxidative stress and indirectly alters gene expressions through reducing peroxynitrite derived from nitric oxide. Med Gas Res. 2011; 1(1): 18.
- Iuchi K, Imoto A, Kamimura N, Nishimaki K, Ichimiya H, Yokota T, et al. Molecular hydrogen regulates gene expression by modifying the free radical chain reaction-dependent generation of oxidized phospholipid mediators. Sci Rep. 2016; 6: 18971.
- Qian L, Cao F, Cui J, Wang Y, Huang Y, Chuai Y, et al. The potential cardioprotective effects of hydrogen in irradiated mice. J Radiat Res. 2010; 51(6): 741-7.
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007; 87(1): 315-424.
- Cardinal JS, Zhan J, Wang Y, Sugimoto R, Tsung A, McCurry KR, et al. Oral hydrogen water prevents chronic allograft nephropathy in rats. Kidney Int. 2010; 77(2): 101-9.
- Itoh T, Hamada N, Terazawa R, Ito M, Ohno K, Ichihara M, et al. Molecular hydrogen inhibits lipopolysaccharide/interferon gamma-induced nitric oxide production through modulation of signal transduction in macrophages. BiochemBiophys Res Commun. 2011; 411(1): 143-9.
- Shi P, Sun W, Shi P. A hypothesis on chemical mechanism of the effect of hydrogen. Med Gas Res. 2012; 2(1): 17
- Sun Q, Kang Z, Cai J, Liu W, Liu Y, Zhang JH, et al. Hydrogen-rich saline protects myocardium against ischemia/reperfusion injury in rats. ExpBiol Med. (Maywood) 2009; 234(10): 1212-9.
- Slezak J, Kura B, Babal P, Barancik M, Ferko M, Frimmel K, et al. Potential markers and metabolic processes involved in the mechanism of radiation-induced heart injury. Can J PhysiolPharmacol. 2017; 95(10): 1190-1203.
- Qin ZX, Yu P, Qian DH, Song MB, Tan H, Yu Y, et al. Hydrogen-rich saline prevents neointima formation after carotid balloon injury by suppressing ROS and the TNF-alpha/NF-kappaB pathway. Atherosclerosis 2012; 220(2): 343-50.
- Chen Y, Jiang J, Miao H, Chen X, Sun X, Li Y. Hydrogen-rich saline attenuates vascular smooth muscle cell proliferation and neointimal hyperplasia by inhibiting reactive oxygen species production and inactivating the Ras-ERK1/2-MEK1/2 and Akt pathways. Int J Mol Med. 2013; 31(3): 597-606.
- Huang CS, Kawamura T, Peng X, Tochigi N, Shigemura N, Billiar TR, et al. Hydrogen inhalation reduced epithelial apoptosis in ventilator-induced lung injury via a mechanism involving nuclear factor-kappa B activation. BiochemBiophys Res Commun. 2011; 408(2): 253-8.
- Fan M, Xu X, He X, Chen L, Qian L, Liu J, et al. Protective effects of hydrogen-rich saline against erectile dysfunction in a streptozotocin induced diabetic rat model. J Urol. 2013; 190(1): 350-6.
- Kura B, Yin C, Frimmel K, Krizak J, Okruhlicova L, Kukreja RC, et al. Changes of microRNA-1, -15b and -21 levels in irradiated rat hearts after treatment with potentially radioprotective drugs. Physiol Res. 2016; 65(1): S129-37.
- Liu GD, Zhang H, Wang L, Han Q, Zhou SF, Liu P. Molecular hydrogen regulates the expression of miR-9, miR-21 and miR-199 in LPS-activated retinal microglia cells. Int J Ophthalmol. 2013; 6(3): 280-5.
- Wei R, Zhang R, Xie Y, Shen L, Chen F. Hydrogen Suppresses Hypoxia/Reoxygenation-Induced Cell Death in Hippocampal Neurons Through Reducing Oxidative Stress. Cell PhysiolBiochem. 2015; 36(2): 585-98.
- VanRooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, et al. A signature pattern of stress-responsive micro-RNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl AcadSci U.S.A. 2006; 103(48): 18255-60.
- Ikeda S, Kong SW, Lu J, Bisping E, Zhang H, Allen PD, et al. Altered microRNA expression in human heart disease. Physiol Genomics. 2007; 31(3): 367-73.
- Jung M, Schaefer A, Steiner I, Kempkensteffen C, Stephan C, Erbersdobler A. Robust microRNA stability in degraded RNA preparations from human tissue and cell samples. Clin Chem. 2010; 56(6): 998-1006.