Research Article
5FU Synergistically Inhibits MCF-7 in Combination with Methylglyoxal
Sonali Ghosh1, Aparajita Pal1 and Manju Ray1,2*
1Department of Biophysics, Bose institute, Centenary Building, India
2Department of Biophysics, GLA University, Mathura, India
*Corresponding author: Manju Ray, Department of Biophysics, Bose institute, Centenary Building, India
Published: 10 Oct, 2017
Cite this article as: Ghosh S, Pal A, Ray M. 5FU
Synergistically Inhibits MCF-7 in
Combination with Methylglyoxal. Clin
Oncol. 2017; 2: 1353.
Abstract
Reduction of toxicity due to uses of conventional chemotherapeutics drugs is a major challenge
in cancer treatment. With this aim we combined 5FU, a widely used chemotherapeutic drug and
methylglyoxal (MG), a non toxic anticancer agent in breast cancer cell line, MCF-7. Treatment
with 5-FU in combination with MG on MCF 7 cells exert synergistic effects in proliferation and
destruction of those cells as determined by MTT, clonogenic assay and scratch wound healing
assay. Results clearly showed that 5FU is more effective at lower doses in presence of MG. Taken
together, our preliminary results revealed that MG can be used in combination therapy to reduce
the concentration of toxic 5-FU against breast cancer cell.
Keywords: Methylglyoxal; 5Fluoro Uracil; Cancer; MCF-7
Introduction
Cancer is one of the leading cause of mortality worldwide having death rate of 9 million in
2016. Among different type of cancers like colon, breast, pancreas, stomach, colorectal, lung there
is an increased surge of breast carcinoma has been observed over several years [1]. Breast cancer is
the second leading cause of cancer death in women with a mortality rate 1 in 37 (about 2.7%) [2].
Chemotherapy is the best option in different types of treatments for advanced stages of tumors.
Existing therapy includes the widespread use of chemotherapeutic drugs such as Doxorubicin,
Paclitaxel, Cisplatin, Etoposide, 5-Flurouracil, Methoxetrate etc [3]. Molecular targeting by these
drugs surely have had a substantial impact on cancer treatment, but patients either eventually
develop resistance to these agents or gets affected by their toxic side effects. Among different types
of chemotherapeutic drugs we have chosen 5-FU as a targeting chemotherapeutic drug in this
current study. The anticancer effects of 5FU already have been studied on different cell lines like
cardiomyocytes [4], colorectal cancer cells [5,6,7] and breast cancers [8] etc. 5-FU is an structural
analogue of pyrimidine .5-FU has some common side effects like nausea, vomiting, headache,
itching, diarrhea etc and also some critical adverse effects like cardiotoxicity [9], vein pigmentation
[10], GI ulceration and bleeding etc.
Reduction of toxic and adverse effects of chemotherapeutic drugs with minimum impact on
efficacy has led to the concept of combination therapy. Combination therapy has a potential for long
term disease modification [11]. Combination of drugs with differing modes of action often attains
a synergistic effect without the toxic side effects associated with a particular drug when used at its
optimal dose. So in this study we want to develop a logical combination of 5-FU and MG to achieve
synergistic killing of cancer cells while avoiding additive toxicity.
The anticancer role of methylglyoxal (MG) has been investigated and established in our
laboratory. Methylglyoxal generally targets malignant cells by affecting glycolysis and mitochondrial
respiration with minimum or no toxicity on normal cells [12-17]. Methylglyoxal is formed as a
byproduct of several metabolic pathways. MG specifically decreases cellular ATP pool by blocking
glycolysis and mitochondrial electron transport chain by inhibiting Glyceraldehyde-3-phosphate
dehydrogenase and mitochondrial complex I respectively of malignant cells. This selectivity of MG
towards malignant cells has been attributed to the differential molecular association of GAPDH
(glyceraldehyde-3-phosphate dehydrogenase) in cancer cells compared to normal cells. It is well
established that GAPDH from normal tissue sources is a homo-tetrameric protein with four
identical subunits of 36 kDa each [18]. By contrast, GAPDH from Ehrlich Ascites carcinoma (EAC)
cells and other cancer cells is a heterodimers having two non-identical subunits of ~33 and 55 kDa
MW respectively [19]. For the first time from our laboratory it has
been identified that GAPDH (33 kDa) is a heterodimer (87 ± 3 kDa)
which interacts with either 55 kDa glucose-6-phosphate isomerase
(GPI) or 55 kDa pyruvate kinase M2 (PKM2) subunit, both of which
are glycolytic enzymes [20].
Depending on the concept of combination therapy which leads
to the reduction of toxicity without compromising the efficacy we
have chosen MG to combine with 5-FU. In this study we chose MCF-
7 as target breast cancer cell line. We study the cumulative effect
of MG with 5-FU to check the cell viability of MCF-7 by MTT cell
viability assay, reproductive capacity of MCF-7 by clonogenic assay,
proliferation and migration capacity by wound healing assay.
Figure 1
Figure 1
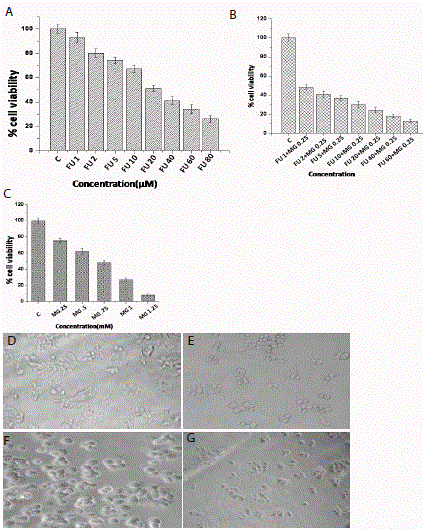
In vitro viability studies of MCF-7 cell line Figure A)- C)
Determination of cytotoxicity (by MTT assay) on MCF-7 cells at 24 h. A)
With increasing concentration of FU (from left to right untreated, 1μM, 2 μM,
5 μM, 10 μM, 20 μM,40 μM,60 μM). B) After 24 hour treatment Cytotoxic
anti-cancer effects of MG at different increasing concentrations (from left to
right untreated MG .25mM, .5mM, .75mM, .1mM, 1.25mM)on MCF-7 cell
line. C) After combining different concentrations of FU with a constant dose
of MG (from left to right untreated, FU 1μM +MG.25mM, FU 2μM+MG.25mM,
FU 5μM +MG.25mM, FU 10μM +MG.25mM, FU 20μM+MG.25mM, FU
40μM+MG.25mM, FU 60μM+MG.25mM). Morphological changes were
viewed by Phase contrast microscopy after 24 hour D) Untreated, E) treated
with 5FU (20μM), F) treated with MG (.5Mm), G) treated with FU+MG
(20μM+.5mM). Concentrations of all the mentioned compounds are indicated
in the X axis of the figure. Each datum is the mean ± standard deviation of
three experiments (n=3). P-value of <0.05 was considered to be significant
in all cases (by one-way ANOVA followed by Dunnett’s multiple comparison
test (to compare between control vs. all the treated groups).
Materials
5-Fluorouracil, MG (40 % aqueous solution, w/v), MTT (3-(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide), Crystal violet solution, 3.7% Formaldehyde, cell culture media and fetal bovine serum were purchased from Sigma Chemical Co., St. Louis, Mo, USA. MCF-7 human breast cancer cell lines were obtained from American Type Culture Collection (ATCC) Manassas, VA, USA). Other chemicals were of analytical grade and obtained from local manufacturers.
Methods
Cell culture
Human breast adenocarcinoma cell line (MCF-7) were
maintained in Roswell Park Memorial Institute medium (RPMI),
supplemented with 10 % fetal bovine serum, penicillin (100 μg/ mL),
streptomycin (100 μg/mL), and gentamycin (100 μg/mL) and grown
in a 37 °C humidified incubator flushed by an air mix containing 5 %
CO2.
Cell viability analysis by MTT assay on MCF‑7 cells
The MTT assay [21] was performed to analyze the cell viability of
MCF-7 cells (breast cancer cell line) with 5-FU supplemented and/
or in combination with MG. Cells in their log phase were seeded
overnight in colorless RPMI in 48 well tissue culture plates such that
each well contained ~15,000 cells. The cells were then incubated with
increasing concentrations of 5- FU (0.25, 0.5, 0.75, 1.0, 1.25, 1.5, and
2.0 μM) and different combinations of MG (1, 2 and 3 mM) for 24
h at 37 °C. The medium was then replaced with 200 μL MTT (0.5
mg/mL) per well and further incubated for 4 h at 37 °C. Then the
insoluble formazan was dissolved in DMSO (dimethyl sulfoxide) (100
μL/ well) and absorbance was measured using a 96-well plate reader
(ELx 800; Biotek, Winooski, VT, USA) at 490 nm. The percentage
of cell viability was calculated compared with the untreated control
(considered as 100 % viable).
Clonogenic assay on MCF-7 cells
Clonogenic assay [22] was performed to analyze the cell
reproductive ability of MCF-7 cells. In 6 well plates around 150
MCF-cells were seeded in each of the wells and cells were allowed to
attach to the plates for 2 h. Then the cells were treated with lower and
higher concentration of 5- FU (.05μM, .25μM) and in combination
with constant dose of MG (.05 mM). For the next 7 days media was
changed on every 2nd day. After 7 days the cells were washed with 1X
PBS and incubated with 3 ml of a mixture of 3.7% formaldehyde in 1X
PBS and 0.5% crystal violet for 30 minutes. The formaldehyde crystal
violet mixture was carefully removed by rinsing with tap water and
plates were allowed to dry at room temperature. Finally, the image
was captured using Gel Doc XR + (Bio-Rad) and colony numbers
were counted.
Wound healing assay
Cells (MCF-7)) were grown to almost 90% confluency in RPMI
media supplemented with FBS. Next, two separate linear scratches
were made by a 200 μl micropipette tip. Wounded monolayers were
washed thrice to remove cell debris, kept in 10% FBS medium and
incubated with 5 FU (10 and 20μM) and MG (.1mM and .25mM)
alone or their combination for 24 h. Images were taken using phase
contrast microscopy.
Results
Effect of 5-FU supplemented with MG on cell viability
To evaluate the effect of 5-FU in combination with MG, varying
doses of 5-FU and MG were tested on MCF-7 cells by MTT assay.
5-FU and MG in combination affected viability of MCF-7 cells in a
dose dependent manner. 5-FU alone at a concentration of 20μM was
able to reduce cell viability to almost 50% (Figure. 1A) and MG at
a concentration of 0.75 mM and 0.25 mM were able to reduce cell
viability to 50% and 20% respectively. (Figure.1B). Treatment with
combination of MG at a concentration of 0.25 mM with varying
concentration of 5-FU has been performed. 50% cell viability was
achieved at a concentration of only 1 μM of 5-FU in combination with
0.25 mM of MG (Figure.1C). In contrast the cell viability was reduced
by approximately 8% by the treatment of 5FU alone. These results
clearly demonstrate that 5-FU, if combined with MG significantly
reduced MCF-7 cell viability compare to 5-FU alone. Phase contrast
microscopic images were also shown morphological changes when
treated with 5FU in combination with MG (Figure.1 D, E, F, and G).
Only 5-FU and MG treated cells were rounded up and many floating
cells in the culture plate were observed. These results clearly suggest a
synergistic effect of 5FU when treated with MG on MCF-7 cells.
Treatment with 5-FU combined with MG significantly
increased cell reproductive death
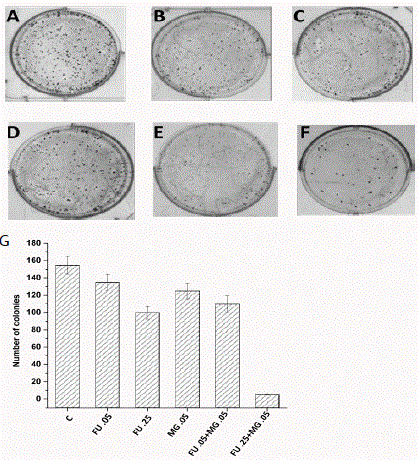
MG supplemented with 5-FU reduced reproductive ability was
detected by clonogenic assay (colony formation). It was performed in
MCF-7 cells using two different concentration of 5-FU (.05μM and
.25 μM) (Figure. 2C and 2D) and a concentration of MG (.05 mM)
alone (Figure. 2B) or in combination (Figure. 2E and 2F) to check the
ability of a single cell to grow into a colony for 7 days. Counting the
colonies significantly demonstrated that 5-FU decreased the colony
numbers when it was treated with MG in MCF-7 cells. At a dose
of 0.25μM of 5-FU and .05mM in combination with MG, very few
colonies were observed than without combination (Figure. 2F).
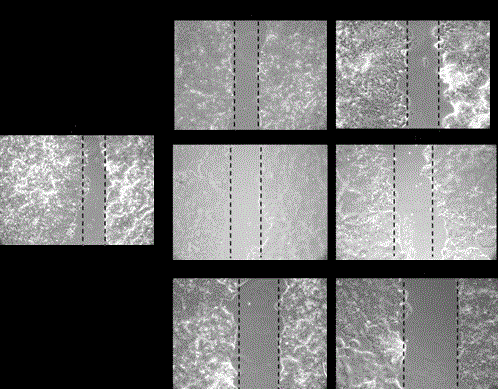
Effect of 5-FU supplemented with MG on cell migration
and growth
To investigate the effect of 5-FU in combination with MG
on cell growth and migration ability, wound healing assay was
performed. MCF-7 cells were treated with varying concentration
of 5-FU (10 μM and 20 μM) (Figure. 3B and 3C) and MG (.1mM
and .25mM) (Figure.3D and 3E) and in combination (MG .1+FU10
and MG.1+FU20) (Figure.3F and 3G) to check the ability of cells to
migrate. Pictures were taken by Phase contrast microscopy after 24
hour of treatment.
Figure 2
Figure 2
Effects of 5Fu and MG on colony formation on MCF-7 cells A)
Untreated, B) Treated with FU (.05 μM), C) Treated with FU (.25 μM),
D) Treated with MG (.05mM), E) FU+MG (.05μM+.05mM), F) FU+MG
(.25μM+.05mM).G) After counting the number of colonies the data is plotted
in a bar diagram. Concentrations of all the mentioned compounds are
indicated in the X axis of the figure. Each datum is the mean ± standard
deviation of three experiments (n=3). P-value of <0.05 was considered to be
significant in all cases (by one-way ANOVA followed by Dunnett’s multiple
comparison test (to compare between control vs. all the treated groups).
Figure 3
Figure 3
Analysis of cell migrating ability after treatment of 5FU and MG in
MCF-7 cells Pictures were viewed by Phase contrast microscopy after 24
hour. A) Untreated, B) treated with FU (10 μM), C) treated with FU (20 μM),
D) treated with MG (.10mM), E) treated with MG (.25mM), F) treated with
FU+MG (10 μM+MG .1), G) treated with FU+MG (20 μM+MG .1).
Discussion
It is evident from the results presented here that MG augments
the anti-cancer effect of 5-FU. This is supported by experiments
performed in vitro. Previously from our laboratory it has been
established that MG specifically targets malignant cells by different
molecular association of GAPDH (glyceraldehyde-3-phosphate
dehydrogenase) in cancer cells compared to normal cells. In this
study the goal was to increase the efficacy of 5-FU in combination
with MG. In this paper, the efficacy of this combination was studied
in human breast carcinoma cell line MCF-7 cells which were
established by cell viability assay by MTT assay. Result showed that
when cells were treated in combination with a constant dose of MG
with different doses of 5-FU, 50% cell viability was attained at a lower
dose of 5-FU in compared to individual treatment with 5-FU. This
result indicates that the combination of MG with 5-Fluorouracil
significantly enhanced the anti-cancer activity of 5-FU. From this
data it also can be assumed that MG decreases the cytotoxic side
effects of 5-FU as it is being used at a very low dose. Phase contrast
microscopic images also corroborate this finding and indicated more
cell death in combination compared to the individual MG and 5FU
treated cells.
5-FU also decreases reproductive growth and production of cells
more efficiently when it was treated in combination with MG. The
result shows that by treating lower and higher dose of 5-FU, number
of colonies had been decreased by 12% and 38%.When cells were
treated with lower and higher dose of 5-FU along with MG, colony
number was decreased by 30% and 95%. The wound healing assay
showed additional decreased motility of MCF-7cells in combination
treatment as indicated by unchanged area of wound. Cells treated
with 5FU and MG were migrated little compared to single treatments.
These data assured that breast cancer cell motility is inhibited more
when MG was used in combination with 5FU. Taken together, our
findings suggested that MG can synergistically increase the efficacy of
5-FU against MCF-7 cells.
Acknowledgements
This research is funded by the Council of Scientific and Industrial Research (CSIR), Government of India; and Department of Science and Technology (DST) INSPIRE scheme , Government of India.
References
- Zackrisson S, Andersson I, Janzon L, Manjer J, Garne JP. Rate of overdiagnosis of breast cancer 15 years after end of Malmö mammographic screening trial: follow-up study. BMJ. 2006;332(7543):689-92.
- T j Key, P K Verkasalo, E Banks. Epidemiology of breast cancer. Lancet Oncology. 2001;2:133-140.
- Pegram MD, Konecny GE, O'Callaghan C, Beryt M, Pietras R, Slamon DJ. Rational Combinations of Transtuzumab With Chemotherapeutic Drugs Used in the Treatment of Breast Cancer. J Natl Cancer Inst. 2004;96 :739- 749.
- Focaccetti C, Bruno A, Magnani E, Bartolini D, Principi E, Dallaglio K, et al. Effects of 5-Fluorouracil on Morphology, Cell Cycle, Proliferation, Apoptosis, Autophagy and ROS Production in Endothelial Cells and Cardiomyocytes, PLoS One. 2015 11;10(2):e0115686.
- S Flis, J Splawinski. Inhibitory Effects of 5-Fluorouracil and Oxaliplatin on Human Colorectal Cancer Cell Survival Are Synergistically Enhanced by Sulindac Sulfide. Anticancer Research. 2009;29:435-442.
- P M De Angelis, D H Svendsrud, K L Kravik, T Stokke. Cellular response to 5-fluorouracil (5-FU) in 5-FU-resistant colon cancer cell lines during treatment and recovery. Molecular Cancer. 2006;5:20-33.
- A Failli, R Consolini , A Legitimo , G Orsini , A Romanini , R Spisni , M Castagna , P Miccoli. Evaluation of in vitro cytotoxicity of oxaliplatin and 5-fluorouracil in human colon cancer cell lines: combination versus sequential exposure. J Biol Regul Homeost Agents. 2015;25:575-88.
- Cameron DA, Gabra H, Leonard R C, Continuous 5-fluorouracil in the treatment of breast cancer. Br J Cancer. 1994;70:120-124.
- F Steger, M G Hautmann, O Kölb. 5-FU-induced cardiac toxicity - an underestimated problem in radiooncology? Radiation Oncology. 2012;7:212.
- R Rao, C Balachandran. Serpentine supravenous pigmentation.A rare vasculo-cutaneous effect induced by systemic 5-fluorouracil. Indian J Dermatol Venereol Lepro. 2010;76:714-715.
- Ghosh S, Bera S, Ray T, Banerjee M, Ray, Methylglyoxal induces mitochondria-dependent apoptosis in sarcoma. Biochemistry (Mosc). 2011;76:1164-1171.
- S Ray, S Biswas, M Ray. Similar nature of inhibition of mitochondrial respiration of heart tissue and malignant cells by methylglyoxal. A vital clue to understand the biochemical basis of malignancy. Mol. Cell Biochem. 1997;171:95-103.
- S Biswas, M Ray, S Misra, D P Dutta, S Ray. Selective inhibition of mitochondrial respiration and glycolysis in human leukaemic leucocytes by methylglyoxal. Biochem J. 1997;323:343-348.
- Ghosh S. Bera S. Ghosal S. Ray A. Basu M. Ray. Differential inhibition/ inactivation of mitochondrial complex I implicates its alteration in malignant cells. Biochem. 2011;76:1051-60.
- Ghosh M, Talukdar D, Ghosh S, Bhattacharyya N, Ray M, Ray S. In vivo assessment of toxicity and pharmacokinetics of methylglyoxal. Augmentation of the curative effect of methylglyoxal on cancerbearing mice by ascorbic acid and creatine. Toxicol Appl Pharmacol. 2006;212(1):45-58.
- Talukdar D, Chaudhuri BS, Ray M, Ray S. Critical evaluation of toxic versus beneficial effects of methylglyoxal. Biochemistry (Mosc). 2009;74:1059- 1069.
- Ferreira-da-Silva F, Pereira PJ, Gales L, Roessle M, Svergun DI, MoradasFerreira P, et al. The crystal and solution structures of glyceraldehyde-3- phosphate dehydrogenase reveal different quaternary structures. J Biol Chem. 2006;281:33433-33440.
- S Bagui, M Ray, S Ray. Glyceraldehyde-3-phosphate dehydrogenase from Ehrlich ascites carcinoma cells. Its possible role in the high glycolysis of malignant cells. Eur J Biochem. 1999;262:386-395.
- Das MR, Bag AK, Saha S, Ghosh A, Dey SK, Das P, et al. Molecular association of Glucose-6-phosphate isomerase and Pyruvate kinase M2 with Glyceraldehyde-3-phosphate dehydrogenase in cancer cells. BMC Cancer. 2016;16:152.
- Twentyman PR, Luscombe M. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br J Cancer. 1987;56(3):279-85.
- Rodríguez-Hernández D, Demuner AJ, Barbosa LC, Heller L, Csuk R. C-Glycosylated cinnamoylfuran derivatives as novel anti-cancer agents. Eur J Med Chem. 2016;115:257-67.
- A D Agostino, A Stellavato, T Busico, A Papa, V Tirino, G Papaccio, et al. In vitro analysis of the effects on wound healing of high- and lowmolecular weight chains of hyaluronan and their hybrid H-HA/L-HA complexes. BMC Cell Biology. 2015;16:19.