Short Communication
Exosome and Its Applications as a Novel Drug Delivery System
Lichun Sun1,2,3,*, Quanyong He1, Zuodong Qin4, Jun Lei1 and Bo Feng5
1The Third Xiangya HospitaI of Central South University, Changsha, China
2Department of Medicine, School of Medicine, Tulane University Health Sciences Center, New Orleans, USA
3Hunan Province Cooperative Innovation Center for Molecular Target New Drug Study, University of South China, China
4Key Laboratory of Comprehensive Utilization of Advantage Plants Resources in Hunan South, Hunan University of Science and Technology, China
5College of Environment and Resources, Xiangtan University, China
*Corresponding author: Lichun Sun, Department of Medicine, School of Medicine, Tulane University Health Sciences Center, New Orleans, USA
Published: 12 Sep, 2017
Cite this article as: Sun L, He Q, Qin Z, Lei J, Feng B.
Exosome and Its Applications as a
Novel Drug Delivery System. Clin
Oncol. 2017; 2: 1346.
Short Communication
For the therapeutics of certain human diseases such as cancers, immune diseases and arthritis,
one of the major problems is that the common drugs used go throughout the whole body, rather
than specifically targeting the pathologic tissues or organs, resulting in severe side effects and less
drug efficacy. There is a pressing need to develop more efficient disease-targeted therapeutics, with
the decrease of side effective toxicity and the increase of drug-targeted specificity. Currently, various
drug delivery systems including antibodies, peptides, proteins, lipids, nanoparticles, polymers,
exosomes, microvesicles have been used for the therapeutic improvement [1-5]. Among them,
exosomes have been exploited, with their unique advantages of small sizes, natural cell-secreted
sources and phospholipid bilayer membrane structures [6,7].
Exosomes were first discovered and reported by Dr. Pan three decades ago [8]. Exosomes are
naturally membrane-derived nanovesicles with their range of 30 nm - 100 nm in diameter. Exosomes
are released by many types of cells such as tumor cells, dendritic cells, endothelial cells, epithelial
cells, T cells, B cells and cortical neurons [9-11], and secreted into the extracellular environments
or physiological fluids. Exosomes can transfer biological messages from donor cells to surrounding
cells or recipient cells in the cell-to-cell communication. Exosomes were observed to have a variety
of different components including proteins, enzymes, antigens, lipids, nucleotides (RNAs and even
DNAs), non-coding RNAs, microRNAs (miRNAs) [12,13]. Their contents are also different in a
cell type-dependent manner. The component difference in exosomes from different cell sources
determines their own critical and particular functions. Exosomes have been demonstrated with
the involvement of various biological, physiological and pathological functions such as immune
response, disease-spreading and tissue homeostasis. For instances, exosomes from tumor cells
participated in tumor growth, tumor progression and tumor metastasis.
The characteristics exosomes have provided potential opportunities for exosome-based
therapeutics. Tumor cell-derived exosomes contain tumor-specific antigens and tumor-associated
biomarkers [12]. These molecules can be targeted for tumor treatments, or applied to recognize
and diagnose these tumors. Also, exosomes might be applied to develop cancer vaccines. Dendritic
cell-derived exosomes carrying various immune responsive factors can be potentially used for
immunotherapy [14]. Exosomes released by virus-infected cells may spread infection over whole
body system. Targeting these exosomes may stop the spreading of viral infection. Exosomes from
neuron cells may be used to pass drugs through un-permeated neuron system and be potential
therapeutics for neuron-associated diseases such as Alzheimer's disease. Due to their unique
characteristics, exosomes have been exploited for human disease diagnosis, therapeutics and vaccine
development. Meanwhile, exosomes have been applied for their broad drug-carrying ability to
deliver different types of drugs or other payloads like proteins, siRNA, and small chemical molecules
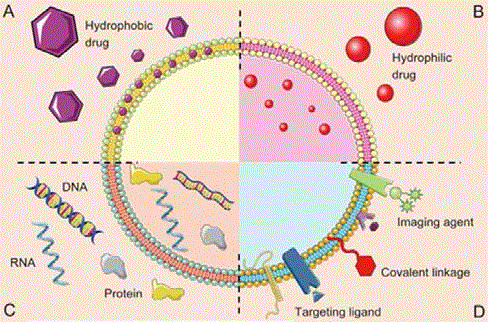
(hydrophobic and hydrophilic) (Figure 1) [6].
Serving as drug delivery vehicles, exosomes display their specific advantages. These exosomes are
natural membrane vesicles with natural cell-released components. Therefore, they are well-tolerated,
with lower toxicity and less immunogenicity [7]. Also, exosomes are small and flexible. They can pass
through the major biological obstacles such as Blood-Brain Barrier
(BBB) and deeply permeate inside tissues [15]. The bi-lipid structures
of exosomes can keep the payloads more stable while avoiding quick
degradation or clearance from body system. Another key advantage
is that the therapeutic exosomes can also come from individual
patient-derived tissue and be used for personalized drug delivery
and therapeutics [7]. More importantly, exosomes from appropriate
source cells are important for driving payloads to targeted tissues due
to the lipid and cell surface components are unique to these exosomes
and important to keep these unique characteristics. Exosomes from
different cell sources have been investigated for the delivery of various
payloads. Certain small molecules such as doxorubicin, paclitaxel,
curcumin have been loaded into exosomes derived from different cell
sources and display the enhanced anticancer efficacy [12]. Various
siRNAs and miRNAs such as BACE1 siRNA, miRNA-21, miRNA-718
[16,17], and display their specific gene-targeting and potent efficacy,
but one of the huge challenges is that these large size nucleotide
molecules are hard to go through cell membranes. Serial studies have
demonstrated that uploading them into exosomes may potentially
be a promising approach to solve this problem. Exosomes also show
their potentials to deliver macromolecular proteins. However, the
exosomal technology has limited cell-targeting ability. Scientists are
still searching for new strategies to improve the specificity of this
technology and the enhanced anti-disease efficacy of the loaded drugs.
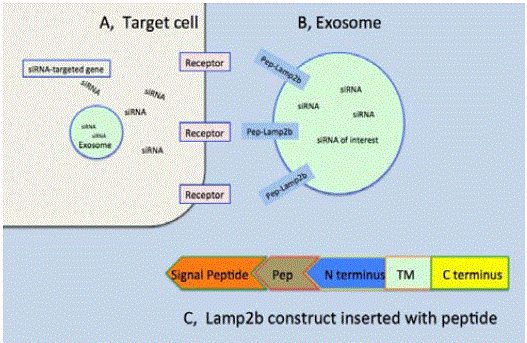
Lamp2b is an exosomal transmembrane protein. The peptide
ligand gene of interest is inserted between the signal peptide and
Lamp2b at its N terminus. The constructs of Lamp2b gene fused
with peptide of interest are transfected into cells that in turn release
exosomes with peptide-Lamp2b expressed on exosomal surfaces. The
payloads such as siRNA or small molecule drugs are then uploaded
into exosomes. The purified exosomes are eventually injected in vivo
in animal models, delivered to the target sites, interact with the cell
surface receptors via the peptide ligands, internalize and release drugs
inside target cells. A, target cell, B, exosome, C, the lamp2b constructs
inserted with peptides of interest. Engineered exosomes expressing
the desired ligands on their surfaces can build ligand-receptor pairing
drug delivery tools to carry drug of interest to targeted sites where the
cell surface receptors are uniquely expressed (Figure 2). Dr. Wood
first designed and established this system [18]. He constructed a
Lamp2b-expressing plasmid and inserted neuron-specific peptide
RVG between signal peptide and Lamp2b at its N-terminus. This
new RVG-Lamp2b construct was transfected into dendritic cells. He
harvested the exosomes released from dendritic cells and expressing
RVG-Lam2b fusion protein and further engineered siRNAs of
interest into these exosomes. This new exosomal system has been
demonstrated to cross the blood-brain barrier and target neurons
via RVG binding to its cognate acetylcholine receptor, implicating it
might be a promising approach to treat neuron-associated diseases.
This valuable exosomal ligand-receptor delivery system can be used
to widely deliver various payloads like siRNAs, small molecules to
targeted sites by inserting different peptide ligands that target different
receptors preferentially expressing in unique tissues. For instances,
some peptide genes like somatostatin, bombesin can be engineered
into the Lamp2b plasmids and the exosomes expressing these
peptides can carry drugs of interest to tissue sites highly expressing
somatostatin receptors and bombesin receptors [19,20] and enhance
the efficacy and specificity of the drugs via the interaction of peptides
and receptors. Exosome may be an exciting next-generation payload
delivery system. However, some questions like side effective toxicity
of exosomes need to be answered. Simply, this exosomal technique
provides us an alternative approach for disease-targeted therapeutics,
diagnosis and precise molecular studies, especially for cancer-targeted
therapy although it is a long way to go.
Figure 1
Figure 1
The different exosomal types of drug delivery systems. A,
hydrophobic drug, B, hydrophilic drug, C, intracellular components (DNA,
RNA and protein), D, membrane components (ligand and receptor) [6].
Figure 2
Acknowledgements
This work was supported by the Zhishan Plan Program Fund from the Third Xiangya HospitaI of Central South University ([2017]15), the Tulane Peptide Research Fund, and the National Natural Science Foundation of China (No. 21606079, No. 21777135).
References
- Wang Y, Wang Q, Wei X, Shao J, Zhao J, Zhang Z, et al. Global scientific trends on exosome research during 2007-2016: a bibliometric analysis. Oncotarget. 2017; 8(29): 48460-48470.
- Sun LC, Coy DH. Somatostatin receptor-targeted anti-cancer therapy. Curr Drug Deliv. 2011; 8(1): 2-10.
- Parslow AC, Parakh S, Lee FT, Gan HK, Scott AM. Antibody-Drug Conjugates for Cancer Therapy. Lancet Oncol. 2016; 17(6): 254-262.
- Fiume L, Manerba M, Di Stefano G. Albumin-drug conjugates in the treatment of hepatic disorders. Expert Opin Drug Deliv. 2014; 11(8): 1203-1217.
- Brazzale C, Mastrotto F, Moody P, Watson PD, Balasso A, Malfanti A, et al. Control of targeting ligand display by pH-responsive polymers on gold nanoparticles mediates selective entry into cancer cells. Nanoscale. 2017; 9(31): 11137-11147.
- Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta pharmacologica Sin. 2017; 38(6): 754-763.
- Lakhal S, Wood MJ. Exosome nanotechnology: an emerging paradigm shift in drug delivery: exploitation of exosome nanovesicles for systemic in vivo delivery of RNAi heralds new horizons for drug delivery across biological barriers. Bio Essays: Bioessays. 2011; 33(10): 737-741.
- Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983; 33(3): 967-978.
- Simhadri VR, Reiners KS, Hansen HP, Topolar D, Simhadri VL, Nohroudi K, et al. Dendritic cells release HLA-B-associated transcript-3 positive exosomes to regulate natural killer function. Plos One. 2008; 3(10): e3377.
- Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992; 176(6): 1693-1702.
- Wang J, Sun X, Zhao J, Yang Y. Exosomes: A Novel Strategy for Treatment and Prevention of Diseases. Front Pharmacol. 2017; 8(2): 300.
- M HR, Bayraktar E, G KH, Abd-Ellah MF, Amero P, Chavez-Reyes A, et al. Exosomes: From Garbage Bins to Promising Therapeutic Targets. Int J Mol Sci. 2017; 18(3).
- Sterzenbach U, Putz U, Low LH, Silke J, Tan SS, Howitt J. Engineered Exosomes as Vehicles for Biologically Active Proteins. Molecular therapy: the journal of the American Society of Gene Therapy. 2017; 25(6): 1269-1278.
- Quah BJ, O'Neill HC. The immunogenicity of dendritic cell-derived exosomes. Blood Cells Mol Dis. 2005; 35(2): 94-110.
- Chen CC, Liu L, Ma F, Wong CW, Guo XE, Chacko JV, et al. Elucidation of Exosome Migration across the Blood-Brain Barrier Model In Vitro. Cell Mol Bioeng. 2016; 9(4): 509-529.
- Guo W, Gao Y, Li N, Shao F, Wang C, Wang P, et al. Exosomes: New players in cancer (Review). Oncol Rep. 2017; 38(2): 665-675.
- Inamdar S, Nitiyanandan R, Rege K. Emerging applications of exosomes in cancer therapeutics and diagnostics. Bioeng Transl Med. 2017; 2(1): 70-80.
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nature biotechnology. 2011; 29(4): 341-345.
- Sun L, Coy DH. Editorial: GPCR-Targeted Drug Development. Curr Drug Targets. 2016; 17(5): 486-487.
- Begum AA, Moyle PM, Toth I. Investigation of bombesin peptide as a targeting ligand for the gastrin releasing peptide (GRP) receptor. Bioorganic & medicinal chemistry. 2016; 24(22): 5834-5841.