Case Report
Disease Free Long Survival after Stump Recurrence and Reoperation of Pancreatic IPMN with Invasive Carcinoma
Emanuele Santoro1*, Paolo Visca2, Roberto Santoro1,2, Pietro Mancini3 and Eugenio Santoro1,2.3
1Department of Oncology, San Camillo City Hospital, Italy
2Department of Oncology, Cancer Institute Regina Elena, Italy
3Department of Oncology, Cristo Re City Hospital, Italy
*Corresponding author: Emanuele Santoro, Department of Oncology, San Camillo City Hospital, Italy
Published: 08 Sep, 2017
Cite this article as: Santoro E, Visca P, Santoro R, Mancini
P, Santoro E. Disease Free Long
Survival after Stump Recurrence and
Reoperation of Pancreatic IPMN with
Invasive Carcinoma. Clin Oncol. 2017;
2: 1342.
Abstract
Re-operation on malignant tumours of the pancreatic stump remaining from a previous resection
for cancer is very rare. The literature reports few cases in which the tumours were mostly made up of
Intraductal Papillary Mucinous Neoplasm (IPMN) sometimes associated with invasive carcinoma
or adenocarcinoma (PDAC).
One of those rare cases has come to our attention: a 62 year old man, anicteric, with a massive
tumour of the head of the pancreas 11 cm x 7 cm x 5 cm, subjected to DCP for IPMN-MD with
isolated areas of invasive cancer, misinterpreted at the beginning as PDAC. Nine years later, with
markers remaining negative, it was re-operated on, a total Pancreatectomy for recurrence of the
same cancer of the pancreatic stump, despite negative lymph nodes. 15 years after the first surgery
and 6 years after the second operation the patient is alive, healthy, diabetic, but the disease free.
The case is an opportunity, through a literature review, to discuss the biological, epidemiological,
therapeutic and prognostic differences between invasive carcinoma, developed on intraductal
papillary mucinous tumours, and tubular primitive pancreatic adenocarcinoma.
Introduction
Cancer of the pancreas is currently the fourth leading cause of death from cancer, and is in
clearly increasing in incidence in both the US and Europe. Despite advances in diagnosis and
treatment, only 4% of cases survive 5 years [1]. Unfortunately, over 80% of cases are presented in
the advanced stages, and are therefore not resectable.
Surgery is the only chance of recovery, but the 5-year survival in different clinical records
varies around 5% [2]. In most cases (80%) the tumour relapses within two years [3]. The median
survival varies from 11 months after surgery alone, to 20 months after surgery plus chemotherapy
[4]. Relapse occurs primarily in the operating region (65%), lymph nodes (17%), liver (11%), in the
peritoneum (7%), with prevalence at the origin of the superior mesenteric artery [5].
The return of the disease on the residual pancreas is rare [6] and in literature only 14 individual
cases are traceable, some of which are considered true relapses and some which are interpreted as
new primitives.
It should also be noted that, regarding pancreatic cancer, there are several histologic types,
no fewer than 13 according to the American College of Pathologists 2005, reported by Lopez [7].
Excluding neuroendocrine forms, adenocarcinoma or ductal carcinoma is the most common and
may originate as such, as a primitive pancreatic cancer (PDAC), or occupy an intraductal papillary
mucinous neoplasm (Inv-IPMN). These last forms have a better prognosis.
Case Report
V.R. 61 years of age, retired, no precedent of diseases or trauma, or surgery, except exanthematous
childhood diseases.
He was hospitalised on 10/12/2001, at the Division of Digestive Surgery of the Regina Elena
National Cancer Institute in Rome, for weight loss and dyspepsia, without jaundice, attributable
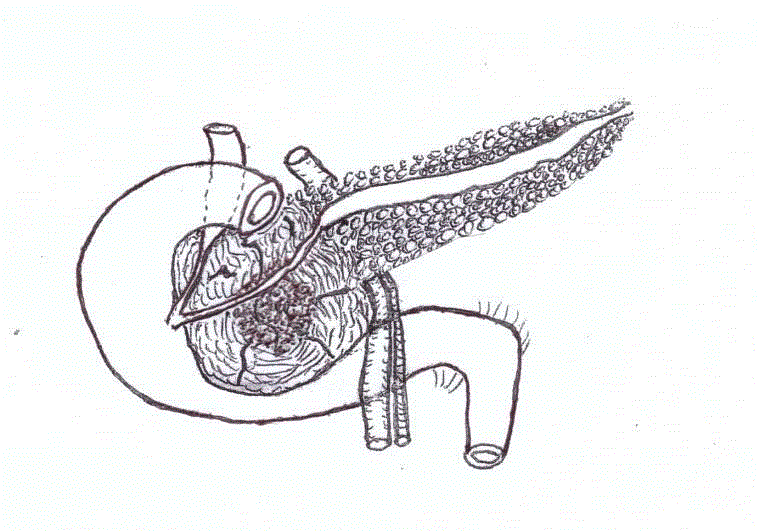
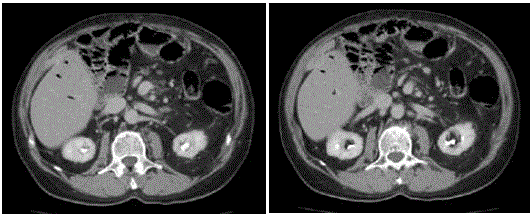
via ultrasound and CT scan (Figure 1) to pancreatic head cancer: "Bulky expansive mass starting
at the head of the pancreas with dimensions of approximately 11 cm x 7 cm x 5 cm; the lesion is
septate with large area of necrosis and with marked contrast graphic
impregnation of the walls. Marked dilatation of duct of Wirsung at
the body-tail section. The common bile duct has mild ectasia in the
distal end and ending in the mass. The lesion has a broad grip with
respect to the gallbladder, gastric antrum, the I and II portions of
the duodenum, the inferior vena cava, the superior mesenteric vein.
Small interaortocaval lymph nodes are present".
At the clinical examination he was found to be a man of medium
height, in a good state of nutrition, not obese, toned, of olive
complexion, with no symptoms of disease other than the abdominal
palpation of a distended gallbladder, hardly painful. MRI confirmed
the CT report. The laboratory tests showed: Hb 13.9 g/dl, GR.
4.850.000, GB 12.100, INR 1.03 , Fibrinogen 408 mg/dl, Blood sugar
151 mg/dl, Azotemia 27 mg/dl, Bilirubin 0.36 mg/dl, GOT 10 UI/L,
GTP 30 UI/L, Proteinemia 5.6 g/dl, CEA 1.8 ng/ml, CA 19 -9 12.4 U/
ml.
He was operated on the 12/12/2001 (Prof. Eugenio Santoro):
Bilateral subcostal incision. voluminous mass of the head of the
pancreas (8 cm x 5 cm) without liver metastasis or peritoneal
carcinomatosis. The mass is tense and elastic, mobile on the back
surfaces, without evident regional lymphadenopathy.
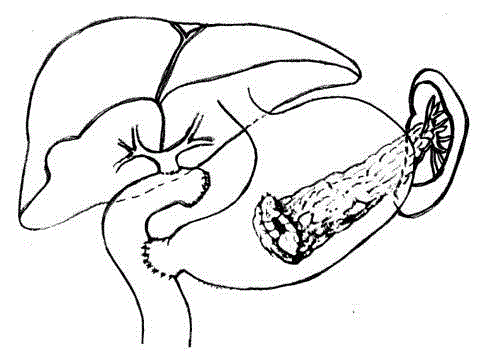
Proceded to pancreaticoduodenectomy, Whipple procedure,
with cholecystectomy and preservation of the pylorus-duodenal
complex. Lymphadenectomy of the hepatic pedicle and the tripod.
Frozen sections of the lymph nodes and slices of section of the bile
duct and the pancreatic remnant tail: all negative. Reconstruction
according to Traverso-Longmire procedure with duodeno-jejunal
anastomosis. Roux-en-Y biliary-jejunal anastomosis. Pancreaticgastric
anastomosis on the posterior wall of the stomach in two
layers of which the inner Wirsung-mucous one had separate stitches
(Figure 2). The postoperative course was complicated by high fevers
and kidney failure, but was cured medically.
In the months and years that followed the patient presented the
same diabetes present at the time of the intervention, sensitive to
insulin. Maintained body weight and regular eating and bowel habits.
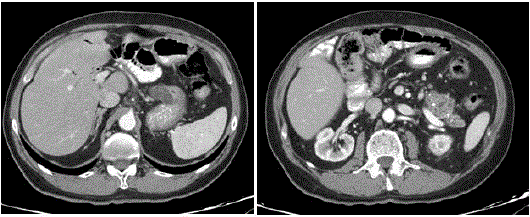
The half-yearly laboratory tests checks-ups were normal, including
CEA and CA19-9 markers (Figure 3). Ultrasound and CT scan were
negative for disease progression or return (Figure 3) with evidence
of calcification and focal ectasia in the residual stump Six years later,
in December 2007, he had a sudden melena with severe anaemia
and unconsciousness. He was hospitalised on 21/12/2008 Ospedale
di Santo Spirito in Rome where he underwent blood transfusions,
TPN and fasting. During the gastroduodenoscopy a duodenal ulcer
was revealed as the site of the bleeding, on which hemostasis was
performed, and also a "evaluation of a gastric lesion 3x4cm with
minimal non-mucous area on the posterior wall" that the histological
examination of the biopsies showed high grade epithelial dysplasia
foci (carcinoma in situ?).
In 2009-2010 subsequent gastroscopies and EUS revealed an
unchanged picture. CEA and CA 19-9 markers negative.
In April 2010 a further gastroscopy with EUS highlighted a
"solid lump on the back midgastric wall with a diameter of 19mm,
occupying mucosa and submucosa with the presence of a central
orifice which emits clear liquid". Histological examination of a biopsy
fragment presumptively showed adenocarcinoma of gastric origin.
He was hospitalised in the Department of General Surgery at the
Ospedale Cristo Re in Rome on 21/06/2010. The abdominal CT scan
with contrast revealed "... outcomes of pancreaticoduodenectomy with
pancreatic-gastric anastomosis. Aerobilia... Irregular increase in size
of the residual pancreas, structurally altered by multiple cystic lesions
and calcifications... Celiac paraortic and paracaval adenopathies".
On 23/06/2010, 8 and a half years after the first operation, a new
laparotomy was carried out on the previous incision"... Viscerolysis,
liver end peritoneum disease free... in the retrogastric evidence of
increased consistency of the pancreas body-tail particularly in the
vicinity of the anastomosis with the stomach. Resection of the blocking
anastomotic mass: wide portion of the rear gastric wall, the entire
pancreatic stump and spleen. Closure of gastric breach. No evidence
of adenopathy" Histological examination of the surgical specimen
describes an "adenocarcinoma of the pancreatic stump infiltrating
the gastric wall and perivisceral adipose tissue. Lymph nodes
unharmed". Simple postoperative course, with early discharge and
return to normal life with progressive full physical recovery, normal
diet, good appetite, stable body weight, suited to body structure and
age, insulin-dependent diabetes well controlled (Figure 4). April 2011
new episode of melena for bleeding duodenal ulcer endoscopically
treated successfully with blood transfusions. Semi-annual check-ups:
laboratory test values within normal limits. Negative markers. CT
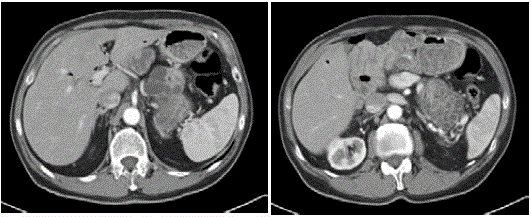
scan with contrast negative for recurrent disease or other condition.
Last CT 2016 (Figure 5) negative for recurrent disease or other
abdominal and thoracic diseases 15 years after the first surgery and 6
after the second, with the patient in good general condition.
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
Figure 6
Discussion
Given the exceptional nature of the case, especially in relation to
the long interval between the first and the second manifestation of the
neoplastic disease and another disease-free survival which amounts
to a total duration of 15 years, it was decided to re-read the CTs and
histological preparations. The first CT of 2001, which resulted in the
first intervention of the pancreaticoduodenectomy on an anicteric
patient, shows "a large lesion of the pancreatic head (11 cm x 5 cm
x 7 cm) with large areas of necrosis of material of lesser consistency,
with multiple divisions", so something that could also be described
as multicystic "with distal dilation of the Wirsung duct", that is a
neoplasm blocking or originating in the main duct, in short, a IPMNMD
which was an absolute indication for surgery, which proved
easier due to the lack of infiltration of surrounding tissues and organs.
In the CTs performed between the first and second surgery, there
was evidence of a residual pancreatic stump after the resection with
irregular parenchyma with irregular margins like cystic or microcystic
evolution of the organ, also in this case probably indicative of a BDIPMN
of peripheral origin.
But it was above all clarifying the reinterpretation of old and
new histological preparations ie those performed on inclusions still
available from the surgical material of the two interventions. The
report: (Dr. P. Visca - IRE)
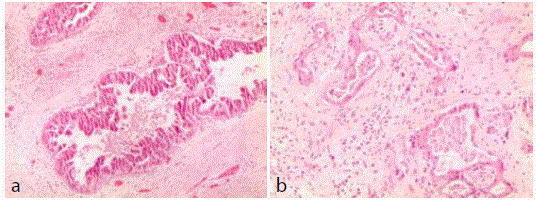
Review of pathological reports of pancreaticoduodenectomy and
performed in 2001: "Pancreatic tissue home to widespread intraductal
papillary mucinous neoplasm (IPMN) with prevailing oncocytic type
aspects and high grade dysplasia (sec. WHO 2010) associated with
invasive cancer foci (G 1-2). All examined peripancreatic lymph
nodes are free of neoplasia (Figure 6a and b).
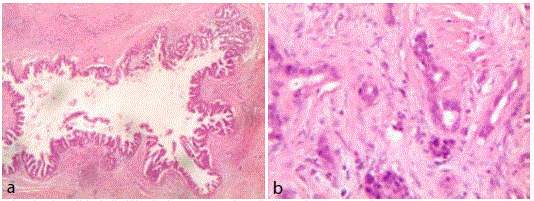
Re-evaluation of pathological slides of body-tail resection of the
pancreas and spleen carried out in 2010: "Pancreatic tissue remnant
home to widespread outbreaks of intraductal papillary mucinous
neoplasm (IPMN) with predominant oncocytic type aspects and
high-grade dysplasia (sec.WHO 2010) in the context of extended
scleroatrophy with hyperplasia of neuroendocrine areas. Finding one
small outbreak of invasive adenocarcinoma (G 2) of 4 mm. Spleen
unharmed (Figure 7a and b).
So in this case it was not a primitive pancreatic adenocarcinoma
of tubular origin (PDAC) and certainly not a gastric adenocarcinoma
as assumed before the second surgery, but an intraductal papillary
mucinous carcinoma of the pancreas associated with outbreaks of
invasive carcinoma ( inv-IPMN) in the first operation in 2001 and
an identical IPMN in the second operation associated with minimal
invasive cancer outbreak. These histologic changes affect the outcome
of the case, justifying the long-term survival, the negativity of markers,
the abscence of metastatic lymph nodes and also the general excellent
condition for all fifteen years of follow up.
In the epicrisis of the case the improper descripton and diagnosis
of the histological examinations reported in medical records is to
be examined, a noteworthy event in medical literature, because the
histological definition of IPMN was highly uncertain at the time
of the 1st intervention in 2001: the interpretation of pancreatic
adenocarcinoma, which was present to only a limited extent, led to
the IPMN, which was almost the entire mass, being ignored. This
initial error probably influenced the examination of subsequent
histological examinations of biopsies and surgical specimens in which
the presence of the papillary mucinous tumour was almost exclusive,
while adenocarcinomal aspects were minimal.
IPMN have been defined in their histogenesis, histological
morphology, biological and clinical behaviour, in the International
Consensus Guidelines of [8,9].
Figure 7
Conclusion
Intraductal Papillary Mucinous Neoplasms (IPMN) have a
potential for malignancy that differs from pre-cancerous lesions
in those with malignant invasive cancer. It is reported that non
inflammatory cystic lesions of the pancreas are very common and
Kimura [10]. Reports them in about half of 300 autopsies. With the
increase of widespread use of imaging asymptomatic cystic tumours
have been systematically discovered. These tumours are divided
into three categories: MD-main duct involving the duct of Wirsung
and with the greatest risk of malignancy, those that originate from
peripheral ducts (BD-branch duct) and mixed types, according to
international guidelines (ICG 2006). According to the subsequent
guidelines (ICG 2012) the following are evidence of a high risk for
cancer: the jaundice of the pancreatic head lesion, an obvious solid
component, a large dilation of Wirsung >10mm. Each of these signs
entails a surgical indication.
From the histological point of view these tumours can be divided
into four stages: mild dysplasia, intermediate, severe and associated
with invasive carcinoma. The first two forms are referred to as benign
-sec [11]. For a full and correct diagnosis above and beyond MRI
and CT scans you can and should practice an endo ultrasonography
with fine-needle aspiration for cytologic examination and assay of
markers, as well as the endoscopic removal of pancreatic juice for
cytologic examination and dosages of carcino-embryonic antigen
[12]. The positivity of these tests is obviously an indication for clinical
intervention; according to Han, the indications for surgical resection
should be limited to the forms with abdominal pain, jaundice and
dilatation of the superior Wirsung to 10 mm.
The Korean study by Han [11] , noted insignificant CEA
values in 290 cases of IPMN, and high values of CA 19.9 with a
mean of 209 U/ml in case with prevalent localisation at the head
of the pancreas. The intervention, according to the offices, was the
pancreaticoduodenectomy or distal pancreatectomy, but in most
patients the histological examination observes mild or moderate
dysplasia for which surgical resection may be considered excessive.
Survival in such cases is sometimes surprising, with definitive
healing and recurrences that are successfully reoperable. For Machado
the 5-year survival for non-invasive forms varies between 77% and
100%, for invasive forms between 27% and 60%, according to a metaanalysis
of the literature [13]. Out of 96 cases of IPMN operated on
from 2006 to 2013, reports 46 as MD, 29 as BD and 21 as mixed,
43 were subjected to DCP, 25 to PD, 6 to segmental and 4 to TP. In
follow-up 14 had developed metastases. The 5-year survival was 96%
in non-invasive forms and 35% in invasive. Of the 18 patients with BD
who were not operated on, all survived and remained asymptomatic.
The largest is the retrospective study from Marchegiani and colleagues
[14] 412 IPMN collected between 1990 and 2013 including 56%
BD, 21% invasive, and 5% PDAC. There was a 17% rate of relapse,
with a 5-year survival of 82% and a 10-year survival of 78%. Only
9 patients required reoperation due to recurrence, all with positive
outcomes, even those with invasive forms. Interestingly, the recent
study by Winter [15] of 76 cases of small IPMN with small invasive
carcinoma, 66% were revealed to be multifocal. There was a local
recurrence in 35% of cases and distant progression in 47%, with an
overall 5-year survival of 59%. The development of neoplasia in the
residual pancreatic stump was studied [16] in 195 cases operated
on for IPMN. The analysis of cases made it possible to identify the
multifocality of the lesions, the adenoma-carcinoma sequence and
the onset of a distinct pancreatic adenocarcinoma (PDAC).
Altogether there were 13 metachronous cases in the stump of
which 6 had severe dysplasia and invasive ca and 7 had concomitant
ductal adenocarcinoma. These recurrences occur with an incidence
of 7.8% at 5 years and 11.8% at 10 years. The case reported by us is
in line with 9 of the 412 described by Marchegiani who underwent
reoperation with positive outcomes for recurrence or new tumours
in the pancreatic stump remaining following previous resection; in
only two cases was it PDAC. Similar activity is reported by Miyasaka,
with 13 IPMN recurrences on the stump out of the 195 operated
on. Like Miyasaka, Winter's 76 cases described the multifocality of
IPMN lesions, their transformation into invasive cancer and the
development of an autonomous adenocarcinoma on the stump,
but which in their case studies represent low percentages. The
case described by us has many similarities with those described by
Marchegiani, Winter and Miyasaka, regarding a relapsed Inv-IPMN
that underwent reoperation with resection and a positive outcome.
However, it has some distinctive features. The first particularity
is that probably the cephalic lesion removed in the first surgery
with the pancreaticoduodenectomy was an IPMN-MD and the
second intervention of a body-tail pancreatectomy was an IPMNBD,
thus confirming the multifocality of cystic lesions. The second
particularity lies in the confirmation in both surgical pieces, of
foci of adenocarcinoma, albeit of T1 size and therefore not yet
widespread, which would confirm the evolution of the IPMM into
invasive carcinoma. The third particularity stems from the fact that
the presence of invasive cancer on the cystic papillary mucinous
tumour was originally interpreted as a simple PADC: this could be a
not uncommon event in large medical records, especially older ones,
and therefore the long survivals reported for pancreatic tumours and
some surprising long-term survival percentages may be related to
incomplete histological diagnosis.
References
- Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011; 378(9791): 607-620.
- Wolfgang CL, Herman JM, Laheru DA, Klein AP, Erdek MA, Fishman EK, et al. Recent progress in pancreatic cancer. CA Cancer J Clin. 2013; 63(5): 318-348.
- Barugola G, Falconi M, Bettini R, Boninsegna L, Casarotto A, Salvia R, et al. The determinant factors of recurrence following resection for ductal pancreatic cancer. JOP. 2007; 8(1): 132-140.
- Rocha-Lima CM. New directions in the management of advanced pancreatic cancer: a review. Anticancer Drugs. 2008; 19(5): 435-446.
- Heye T, Zausig N, Klauss M. CT diagnosis of recurrence after pancreatic cancer: is there a pattern? World J Gastroenterol. 2011; 17(9): 1126-1134.
- Akabori H, Shiomi H, Naka S, Murakami K, Murata S, Ishida M, et al. Resectable carcinoma developing in the remnant pancreas 7 years and 10 months after distal pancreatectomy for invasive ductal carcinoma of the pancreas: report of a case. World J Surg Oncol. 2014; 12: 224.
- Lopez Massimo. Oncologia Medica Pratica. III Edizione p.1767. Ed. Universo, Rome. 2010.
- Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006; 6(1-2): 17-32.
- Goh BK, Lin Z, Tan DM, Thng CH, Khor CJ, Lim TK, et al. Evaluation of the Fukuoka consensus Guidelines for intraductal papillary mucinous neoplasms of the pancreas. Results from a systematic review of 1,382 surgically resected patients. Surgery. 2015; 158: 1192-1202.
- Kimura W, Nagai H, Kuroda A, Muto T, Esaki Y. Analysis of small cystic lesions of the pancreas. Int J Pancreatol. 1995; 18(3): 197-206.
- Han DH, Lee H, Park JY, Kwon W, Heo JS, Choi SH, et al. Validation of international consensus guideline 2012 for intraductal papillary mucinous neoplasm of pancreas. Ann Surg Treat Res. 2016; 90(3): 124-130.
- Machado NO, Al Qadhi H, Al Wahibi K. Intraductal Papillary Mucinous Neoplasm of Pancreas. J Med Sci. 2015; 7(5): 160-175.
- Tian X, Gao H. Surgical treatment and prognosis of 96 cases of intraductal papillary mucinous neoplasms of the pancreas: a retrospective cohort study. Int J Surg. 2015; 13: 49-53.
- Marchegiani G, Mino-Kenudson M, Ferrone CR, Morales-Oyarvide V, Warshaw AL, Lillemoe KD, et al. Patterns of Recurrence After Resection of IPMN: Who, When, and How. Ann Surg. 2015; 262(6): 1108-1114.
- Winter JM, Jiang W, Basturk O, Mino-Kenudson M, Fong ZV, Tan WP, et al. Recurrence and Survival After Resection of Small Intraductal Papillary Mucinous Neoplasm-associated Carcinomas (≤20 mm Invasive Component): A Multi-institutional Analysis. Ann Surg. 2016; 263(4): 793-801.
- Miyasaka Y, Ohtsuka T, Tamura K, Mori Y, Shindo K, Yamada D, et al: Predictive Factors for the Metachronous Development of High-risk Lesions in the Remnant Pancreas After Partial Pancreatectomy for Intraductal Papillary Mucinous Neoplasm. Ann Surg. 2016; 263(6): 1180-1187.