Editorial
Teaching Old Drugs New Smart Tricks: Rethinking Cancer Therapeutics via Bioconjugate Technologies
Mohamed Ismail Nounou*
Department of Pharmaceutical Sciences, School of Pharmacy, University of Saint Joseph, Hartford, USA
*Corresponding author: Mohamed Ismail Nounou, Department of Pharmaceutical Sciences, School of Pharmacy, University of Saint Joseph, Hartford, USA
Published: 14 Jun, 2017
Cite this article as: Nounou MI. Teaching Old Drugs New
Smart Tricks: Rethinking Cancer
Therapeutics via Bioconjugate
Technologies. Clin Oncol. 2017; 2:
1337.
Editorial
Targeting cancer cells while circumventing non-cancerous cells is the main challenge in
cancer therapy. Over the past two decades, numerous systems and strategies have been designed
for overcome this problem. Initiatives to overcome this problem either involved the design and
development of novel new chemotherapeutic agents or chemical or physical modifications of
currently used chemotherapeutic agents [1].
For the past couple of decades, academic research has been mainly focusing on novel carrier
systems and nano particulate colloidal technologies for drug delivery, such as nanoparticles,
nanospheres, vesicular systems, liposomes or nanocapsules to impart novel functions and targeting
abilities to currently used chemotherapeutic agents. Such efforts aided in the creation of newly
marketed products such as Doxil® (Doxorubicin encapsulated in PEGylated liposomal vesicles) in
the market [2,3]. Such systems provide the tools to custom design a superior drug delivery system,
impart novel functions to old drugs such as longer half-life and stealth properties (as in the case of
Doxil®), and provide them with either passive or active targeting properties via grafting the carrier
system with targeting moieties and/or imaging agents or another drug within the same carrier
system [4]. Such technologies opened the gate towards more sophisticated and effective multi-acting
platform(s) which can offer site-targeting, imaging, and treatment using a single multi-functional
system [5]. Unfortunately, such technologies are faced with major problems including high cost,
low stability profile, short shelf-life, and poor reproducibility across and within production batches
leading to harsh bench-to-bedside transformation. Major process and formulation development
concerns exist with respect to scale-up processes of complex nanoparticluate carriers. Most of the
reagents and inactive moieties in the formulation of such novel therapeutic systems are not included
in the FDA approved Inactive Ingredient Database (IID).
Currently, pharmaceutical industry along with academic research are investing heavily in
bioconjugate structures. Bioconjugate technologies offer an appealing and advantageous alternative
to nanoparticulate delivery systems with all its flexible benefits when it comes to custom design
and tailor grafting along with avoiding most of its shortcomings. Bioconjugates offer the flexibility
of custom designing personalized products. Bioconjugates facilitate simple and easy drug (active
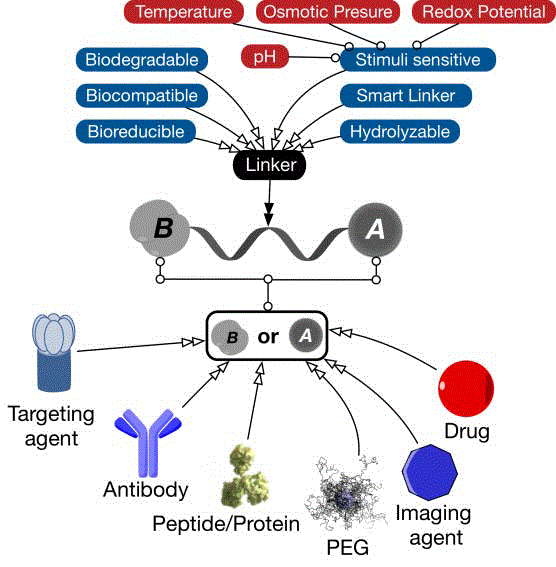
pharmaceutical ingredient) conjugation, using various smart
biocompatible, bio-reducible, or biodegradable linkers, to targeting
agents, PEG layer or another drug (Figure 1). Such technology enables
the formation of smart multi-functional platform(s) offered by
nanoparticulate carriers. Furthermore, conjugates are still considered
chemical compounds.
This fact simply allows the use of traditional analytical
and manufacturing technologies in the characterization and
manufacturing of traditional active pharmaceutical ingredients
offering high probability for their successful transition from bench-tobedside.
Moreover, the final formulation could be a simple injectable
or solid formulation, which offers long shelf-life and enhanced stability
profile. The main aim of bioconjugation is to form a stable biologicallycleavable
covalent link between two molecules, at least one of which
is a biomolecule [6]. Bioconjugation aims to increase stability, protect
drug from proteolysis, or to enhance the targeting properties of the
delivery system. Inspite of the historic fact that bioconjugates are
older than nanoparticles, research is currently being diverted back to
it. The design of a useful bioconjugate will depend mainly on its use,
purpose and the desired properties needed. Thus one could choose a
suitable molecule and suitable cross-linker to form the bioconjugate
[7]. Bioconjugates can be synthesized by using cross linkers (couplers)
that have been designed for this purpose, or by using a reactive group
on one of the two molecules to facilitate the reaction [6]. Most of
these reactions couplers are used to enhance the conjugation yield
such as 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC,
EDAC or EDCI) and N,N'-Dicyclohexylcarbodiimide (DDC). EDC
is often used in combination with N-Hydroxysuccinimide (NHS) for
the immobilisation of large biomolecules [6]. Bioconjugation can also
be carried out by using a secondary activating agent, that forms an
intermediate reactive group on one of the two molecules and thus
facilitates the coupling reaction. Thus the key to forming a successful
bioconjugate is choosing the suitable cross linker between the
molecules [6]. Bioconjugation technologies can aid in creating safer,
cheaper, stable, and effective novel therapeutics. It can also be a ratelimiting
step in reinventing old drugs and imparting new functions
to them that would enhance their target ability, pharmacokinetic and
pharmacodynamic parameters, and their overall formulation patient
compliance, easing their transition to market. A major focus should
be the transformation of such novel bioconjugates’ technologies
from bench-to-bedside. The use of click chemistry, bioconjugation
technologies, ligand post-insertion, and labeling techniques need to
be extensively researched for ease of scale up and proper bench-tobedside
transformation.
Figure 1
References
- Lammers T, Kiessling F, Hennink WE, Storm G. Drug targeting to tumors: Principles, pitfalls and (pre-) clinical progress. J Control Release. 2012; 161(2): 175-187.
- Muggia FM. Doxil in breast cancer. J Clin Oncol. 1998; 16(2): 811-812.
- Porche DJ. Liposomal doxorubicin (Doxil). J Assoc Nurses AIDS Care. 1996; 7(2): 55-59.
- Tagami T, Ozeki T. Recent trends in clinical trials related to carrier-based drugs. J Pharm Sci. 2017; 106(9): 2219-2226.
- Cagel M, Tesan FC, Bernabeu E, Salgueiro MJ, Zubillaga MB, Moretton MA, et al. Polymeric mixed micelles as nanomedicines: Achievements and perspectives. Eur J Pharm Biopharm. 2017; 113: 211-228.
- Hermanson GT. Bioconjugate Techniques (Third Edition). Third ed. 2013.
- Duncan R. Polymer conjugates as anticancer nanomedicines. Nature reviews Cancer. 2006; 6(9): 688-701.