Research Article
Microsatellite Instability in Chronic Myeloid Leukemia Using D17S261 and D3S643 Markers - A Pilot Study in Western India
Trupti N Patel1*, Manali Chakraborty2 and Priyanjali Bhattacharya1
1Department of Integrative Biology, Vellore Institute of Technology, Vellore, India
2Department of Biomedical Genetics, Vellore Institute of Technology, Vellore, India
*Corresponding author: Trupti N Patel, Department of Medical Biotechnology, Vellore Institute of Technology University, Vellore, India
Published: 01 Aug, 2017
Cite this article as: Patel TN, Chakraborty M, Bhattacharya
P. Microsatellite Instability in Chronic
Myeloid Leukemia Using D17S261
and D3S643 Markers - A Pilot Study
in Western India. Clin Oncol. 2017; 2:
1322.
Abstract
The incidence of microsatellite instability in chronic myeloid leukemia was investigated in a series of 10 patients confirmed by t(9;22). MSI found in clinical CML samples, directed a variation in (AC) n repeats for the markers screened. The future perspective of this study is to establish diagnostic and prognostic significance of MSI in haematologic malignancies.
Introduction
Genomic instability deals with the understanding of genome wide changes that lead to functionally impaired genomic regions and complex disease conditions like cancer. Chromosomal instability is visible changes in the chromosomes whereas Microsatellite Instability (MSI) is usually accompanied by variations in the nucleotide repeats as a result of faulty mismatch repair (MMR) system that includes genes such as MSH2, MLH1, MSH6, PMS1, PMS2, MLH3 [1,2]. Microsatellites are highly polymorphic, short repeat nucleotide sequences spread throughout the genome. The repetitive di- and tri-nucleotides are sited about once in every 40kb of DNA between or within the genes. Simple microsatellite sequences can be (A) n, (CA) n, (GATA) n, where n lies between 5-30 [3]. In humans, dinucleotide repeat of cytosine and adenine is the most common microsatellite region and in normal cells, the number of repeat units is maintained with high accuracy. The objective of our study is to check for MSIs in Ph+ Chronic Myeloid Leukemia (CML) patients so that microsatellite instability testing can be incorporated in diagnostics of haematologic malignancies along with other cytogenetic and/or molecular assays.
Materials and Methods
Based on previous reported data, microsatellite instability was investigated and analyzed in D17S261 (17p12-11.1) and D3S643 (3p21.3) markers by comparing electro preprograms for both normal and cancerous samples. The diagnosis of ten CML patients was based on standard clinical criteria and cytogenetic report of t (9;22) positive in all the samples. The reverse primers of both the markers were tagged with fluorescent dyes FAM (D17S261) and HEX (D3S643) respectively to carry out single reaction (Table 1). Standard PCR was performed makinga reaction volume to 50μl. The annealing temperatures for FAM and HEX tagged markers were set at 50° C and 60° C respectively. PCR was followed by Fragment Analysis with a reaction mixture of - 0.5μl PCR product, 0.5μl Liz500, and 9μl HI-DI formamide by capillary electrophoresis (3500 genetic analyzer instrument) and the data was evaluated using Gene Mapper software.
Results
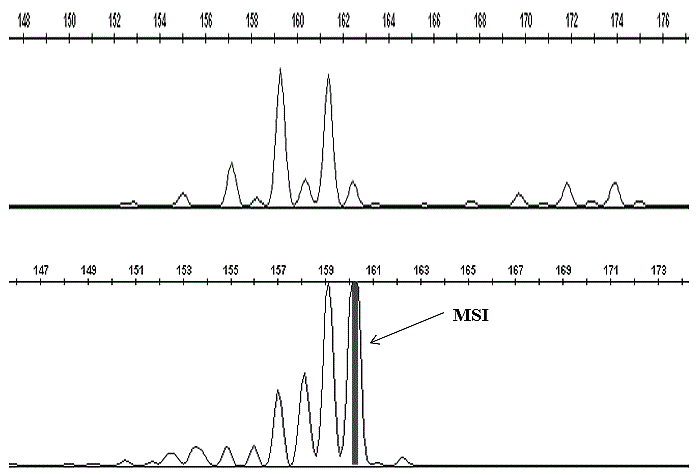
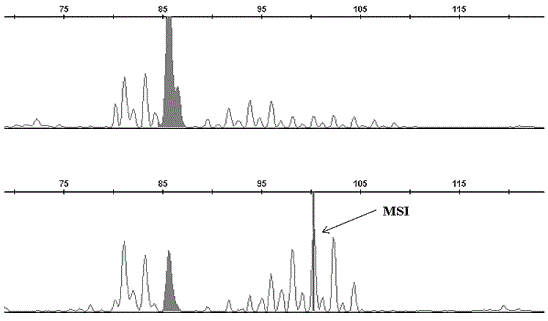
MSI was seen to be present in all the patients studied for (AC) repeat in 159 bp -161 bp region for D17S261 marker (Figure 1) whereas in D3S643 marker, four patients did not show any variation in comparison with controls. However, six patients showed variability near 94 bp, 100 bp and 101 bp indicating instability. A common peak of 85 bp was constant for 10 patients in D3S643 marker and this region may be considered as a non-vulnerable region (Figure 2).
Discussion
Our findings demonstrate a clear occurrence of microsatellite instabilities in (AC) repeat markers D17S261 and D3S643formost of the leukemic patients hence correlating MSIs with plausible low expression of MMR genes. For D17S261 marker, a predictive microsatellite variation on 17p might indicate complete or partial functional inactivity of tumor suppressor gene p53 (17p13.1) as because this gene is in the closest vicinity for the marker screened. Thus, it can be assumed that among DNA mismatch repair genes, a close interaction between MSH2 and p53 might aid to CML progression followed by metastasis and mortality since the promoter region of MSH2is seen to have a response element for p53 thereby regulating its expression [4-7]. Fragment analysis reports of D3S643marker showed microsatellite instability in six patients. Two different peak patterns were recorded; one at 85 bp region which was constant (non-vulnerable) for each sample and another peak around 100 bp region that seems to be vulnerable and might play a role in transition in CML. Moreover, presence of MSI for D3S643 marker indicates an alteration in one of DNA repair genes MLH1 (3p21.3).
Figure 1
Figure 1
Complete fragment analysis result of control (top panel) and patient (bottom panel) for D17S261 marker.
Figure 2
Figure 2
Complete fragment analysis result of control (top panel) and patient (bottom panel) for D3S643 marker.
Conclusion
Researches on microsatellite instabilities in haematologic malignancies are limited in number and results testified indicated this phenomenon to be infrequent and it may not go along with cytogenetic rearrangements since there are minorities of cases where patients do not show any genetic aberrations but are symptomatic for leukemia or lymphomas. In such exceptional events, clinicians may take up MSI testing as a routine investigation along with cytogenetic analysis or immunohistochemistry for a better clinical management and systematic treatment in leukemia and lymphoma.
References
- Wada C, Shionoya S, Fujino Y, Tokuhiro H, Akahoshi T, Uchida T, et al. Genomic instability of microsatellite repeats and its association with the evolution of chronic myelogenous leukemia. Blood. 1994; 83(12): 3449-3456.
- H Ogün Sercan, Zeynep Y Sercan. Microsatellite instability is a rare phenomenon in transition from chronic to blastic phase chronic myeloid leukemia. Turkish J Cancer. 2001; 31(9): 63-71.
- Nanna Claij, Hein te Riele. Microsatellite instability in human cancer: A prognostic marker for chemotherapy? Experimental Cell Research. 1999; 246(2): 1- 10.
- Yano M, Hamatani K, Eguchi H, Hirai Y, MacPhee DG, Sugino K,et al. Prognosis in patients with hepatocellular carcinoma correlates to mutations of p53 and/or hMSH2 genes Eur J Cancer. 2007; 43(6): I092- I100.
- Zhu YM, Das-Gupta EP, Russell NH. Microsatellite instability and p53 mutations are associated with abnormal expression of MSH2 gene in adult acute leukemia. Blood. 1992; 94 (2): 733- 740.
- Daniele Zink, Christoph Mayr. Association of p53 and MSH2 with recombinative repair complexes during S phase. Oncogene. 2002; 21(31): 4788-4800.
- Ben-Yehuda D, Krichevsky S, Caspi O, Rund D, Polliack A, Abeliovich D, et al. Microsatellite instability and p53 mutations in therapy related leukemia suggest mutator phenotype. Blood. 1996; 88(11): 4296-4303.