Research Article
The Pioneering Hypotheses of Exercise Effects on Tumor Growth - Systematic Review
Claudia Arab1,2*, Tânia Brusque Crocetta3, Patricia Morgana Rentz Keil2, Renata Thaís de Almeida Barbosa3, Carlos Bandeira de Mello Monteiro4, James Tonks5, Thais Massetti6 and Alexandro Andrade2
1Departamento de Medicina (Cardiologia), Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, SP, Brasil
2Centro de Ciências da Saúde e do Esporte, Universidade do Estado de Santa Catarina – UDESC, Florianópolis, SC, Brasil
3Laboratório de Delineamento de Estudos e Escrita Científica, Departamento de Saúde da Coletividade, Disciplina de Metodologia Científica, Faculdade de Medicina do ABC, Santo André, SP, Brasil
4Escola de Artes, Ciências e Humanidades da Universidade de São Paulo, EACH – USP, São Paulo, SP, Brasil
5Haven Clinical Psychology Practice, University of Exeter Medical School, Cornwall, UK
6Faculdade de Medicina da Universidade de São Paulo – Departamento de Fonoaudiologia, Fisioterapia e Terapia Ocupacional – FMUSP, São Paulo, SP, Brasil
*Corresponding author: Claudia Arab, Departamento de Medicina (Cardiologia), Escola Paulista de Medicina, UniversidadeFederal de São Paulo, 715 Napoleão De Barros St., São Paulo, SP 04024-002, Brazil
Published: 10 Jul, 2017
Cite this article as: Arab C, Crocetta TB, Keil PMR, de
Almeida Barbosa RT, de Mello Monteiro
CB, Tonks J, et al. The Pioneering
Hypotheses of Exercise Effects on
Tumor Growth - Systematic Review.
Clin Oncol. 2017; 2: 1313.
Abstract
Introduction: Over the past seventy years, the relationship between physical exercise (PE) and
cancer has been researched extensively, but the biological changes associated with PE and its
probable influence on the tumor growth and patient survival are still uncertain.
Aim: The objective of this study was to identify and analyze pioneering hypotheses in relation to the
effect of PE and tumor growth in experimental animal models.
Methods: We conducted a descriptive historic review of the literature through PRISMA protocol
by Virtual Health Library on electronic databases MEDLINE, LILACS, IBECS and SciELO. The
inclusion criterion was experimental studies that submitted animals to exercise and sought to
explicate the relationship between exercise and tumor growth through biological mechanisms.
Results: The pioneering hypotheses indicated that PE effects on tumor growth were due to
energy-related metabolic factors which inhibit tumor growth. PE inhibits tumor growth through
(a) alternative consumption of energy otherwise available in the development of tumor cells or
(b) secretion of substances produced by muscle contraction in fatigue. However, there are other
determining factors related to life experiences.
Conclusion: There is preliminary evidence of PE being beneficial in tumor inhibition, but
acknowledge that the mechanisms involved in the effects of exercise on tumor growth remain
uncertain, possibly due to the wide variety of tumor types and biological intra-individual variation.
Keywords: Neoplasms; Cancer; Physical exercise
Introduction
Cancer is a major public health problem worldwide and is the second leading cause of death in
the United States. In 2016, the 1,685,210 estimated new cases of invasive cancer were reported in
the US [1]. Cancer is widely considered to be a cell-autonomous genetic disease that results from
alterations in oncogenes, tumor-suppressor genes and genome-stability genes [2]. Basically, tumor
cells acquire a common set of properties including unlimited proliferation potential, self-sufficiency
in growth signals, and resistance to anti-proliferative and apoptotic cues [3].
Over the past seventy years, researchers have investigated the relationship between cancer and
physical exercise (PE) [4]. In the last three decades, changes in the areas of oncology and kinesiology
have resulted in rapid advances in interventions for cancer survivors. Some of this work has focused
upon PE in prevention and rehabilitation [5].
Several experimental studies of interventions have been developed with human beings. These
attests to various PE protocols, and include evidence of feasibility and physical exercise guidelines
for cancer patients and survivors [4,6-13]. However, the primary
effects of exercise on tumor growth and progression remain relatively
poorly understood. Primary effects that have been identified include
biological changes in tumor cells or the tumor micro-environment
that are a direct consequence of exercise, such as alteration in
oxidative status and gene expression [14]. In animal models, exercise
has been show influence modulation of the micro-environment of
mammary tumor development [15]. The authors found that exercise
training promoted tumor vascularization and growth in tumors
with higher volume, but reduced the number of mammary tumors
and aggressiveness, and increased latency period in female Sprague–
Dawley rats. This study indicates that, although involved complex
and poorly understood variables (such as tumor volume), there is a
preliminary indication of the benefits of PE [16].
We consider that the elucidation of the molecular mechanisms
underlying the association between exercise and cancer is of
paramount importance in optimizing the safety and efficacy of
exercise in cancer control [17]. It may have important implications
for inhibiting tumor metastasis and improving the efficacy of
conventional cancer therapies [17]. The molecular mechanisms
underlying the exercise-tumor-genesis relationship are complex, and
proposed explanations remain speculative [17].
In the context of the recent and rapidly accruing research in this
area, it is timely to review previous findings and addressing future
directions. On this basis, our aim was to explore the pioneering
hypotheses and related findings published on the effects of exercise on
tumor growth in experimental animal models through a systematic
review.
Table 1
Table 1
Study, objectives, methods, main results and pioneering hypothesis of experiments with animal models on the effects of exercise on development of selected
cancer in MEDLINE, LILACS, and SciELO IBECS.
Method
This is a descriptive historic review that analysed the primary
hypotheses published in literature about the effect of exercise on
tumor growth in experimental animal models. We followed the
Preferred Reporting Items for Systematic reviews and Meta-Analyses
(PRISMA) protocol on MEDLINE, LILACS, IBECS eSciELO databases
throughVirtual Health Library – Regional Portal. We searched
articles published from 1981, the point from which the original and
first study with cancer patients and exercise was undertaken [5]. The
primary search terms used were “cancer” and interchangeable terms
such as “tumor” and “neoplasm”, combined with secondary terms
“physical exercise”, “exercise” and “physicalactivity” in title, abstract
or subject.
The inclusion criterion was experimental studies that submitted
animals to exercise and sought to explicate the relationship between
exercise and tumor growth through biological mechanisms. The
search also included references of selected studies and in Cancer
Research, Science e Psychological Reports journals.
Results
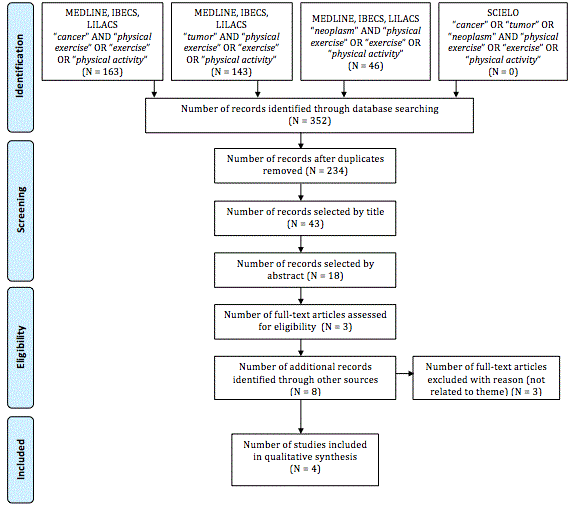
We initially identified 352 articles. Four pioneer experimental
studies using animal models, and which focused upon the relationship
between exercise and tumor growth were selected for this review (see
Figure 1).
PRISMA model adapted from Liberati et al. [18].
The primary hypotheses used in animal models that considered
the relationship between exercise and cancer were published between
1944 and 1962. The studies used mice and rats as models and are
described below (see Table 1).
Rusch, Kline - 1944
The first publication on the subject of exercise effects on tumor
growth was Rusch and Kline [19] study in 1944. The authors
investigated the influence of forced exercise on tumor growth rates
in mice bearing transplantable tumors. The authors believed it would
be possible to inhibit tumor growth by subjecting animals to forced
exercise. The exercise, and consequently energy bodily requirements,
were found to inhibit the neoplastic cell development because there
remained little, or no excess energy. Young adult ABC male mice were
divided into two groups: one group was subjected to forced exercise,
and the other group was control situation. After a preliminary 1
or 2 weeks-period of exercise, Rusch and Kline21 subcutaneously
inoculated transplantable fibrosarcoma on the abdominal region in
all mice. The original fibrosarcomas were obtained from the ear of a
mouse, which received continued ultraviolet irradiation.
The experiment was conducted with two series of mice. In the
first series, 100 mice were divided into two groups of 50 each (exercise
and control). Exercise was induced by rotating cages for 16 hours
continuously and was followed by a rest period for the remaining 8
hours of the day. In this series, the exercise was started 1 week before
the mice were inoculated with the sarcoma. In the second series,
composed of 40 mice in each group, the exercising group was rotated
for 2 hours at a time with an alternate 1-hour rest period throughout
the 24-hour period. The exercise was started 2 weeks before tumor
inoculation and continued for the duration of the experiment. The
results showed more retardation of tumor growth trend in exercise
groups than in control groups. In the first series, the tumor size was
0.43 to 2.42 units, while the second series was 0.16 to 1.53 units. The
control groups showed improvement of 0.58 to 3.21 and 0.28 to 2.33
units, respectively. The hypothesis of Rusch and Kline was confirmed,
and they concluded it was possible to inhibit the tumor growth in
animals through exercise because of the utilization of excess energy
[19].
Rashkis - 1952
The second identified published study was Rashkis [19], in
1952. The study investigated the effect of stress (forced swimming)
on inoculated tumor inhibition in Swiss mice. The hypothesis was
that organisms under stress would show less tendency to develop
experimentally produced tumors than normal controls because of
stress catabolic (inhibitor effect). In the first tests, 25-young adult
male Swiss albino mice made up the experimental group. These were
submitted to 17 days of forced swimming with progressive duration
(i.e. from one and a half hour to four hours daily, with 15 minutes
increased per day). After intraperitoneally tumor inoculation the
mice were forced to swim 34-hours during 14 days.
The duration of sessions was decreased from four and a half
hours to 15 minutes daily. Discontinued swimming was necessary
in the experimental group because of the tumor growth, which also
increased the danger of mice deaths by drowning. The average of
survival time in the experimental group was 18 days, 20% more than
control group. A second experiment consisted of sarcoma inoculation
by methylcholanthrene in mice and investigated the stress effect in
different ways. The experimental group was forced to swim until
exhaustion (i.e. during 5 to 36 days). This method of exercise was
tested in periods according to tumor inoculation: pre, post, and both
pre and post. The post-inoculation group was forced to swim for
less time and showed the greatest survival rate. The group that was
submitted to more time of swimming was exhausted and developed
a leucocytosis infection. Thus, the results showed that there was a
likelihood that imposed stress (i.e. exercise) promoted the inhibition
on tumor growth [20].
Hoffman et al. - 1962
In 1962, Hoffman et al. [21] tried to determine whether a
mitosis-inhibiting substance produced by fatigued muscles could
inhibit tumor growth in male Wistar rats. The animals were divided
into two exercise groups: (I) with no substance injection and (II)
with fatigue substance (F-Substance) injection. A third group was
composed of fifteen tumor-bearing animals confined in individual
cages to keep activity at a minimum as a control condition. The
exercise experiment consisted of Walker 256 tumors transplanted by
injection subcutaneously into the right thigh of 24 animals that were
posteriorly exercised daily for 3 weeks. The experiment consisted
of: (a) the animal being first conditioned by electric shock to run
continually in a 20-foot runway; (b) after a brief rest, the animal was
forced to swim, starting with a 20-minute period and increasing by 20
minutes per day to a maximum of 4 hours; (c) the animal was placed
in a revolving drum where it ambulated each night for a recorded
distance of 5.4 miles over 12 hours.
The injection experiments by Hoffman et al. [21] used male
Wistar and Sprague-Dawley rats that received inoculations of the
Walker 256 tumor or the Murphy lymphosarcoma. The F-substance
was produced through electric stimulation on the rectus femoral
muscle of the rat. This was applied to produce contraction to the
point of fatigue. The substance was injected subcutaneously when
the smallest tumor in the control animals reached a size of 2 cm. in
diameter, 7-10 days following transplantation [21].
The results showed that in the (a) experiment, the tumor weight
in control rats exceed tumor weight of the exercised animals in every
instance. In several cases, there was complete tumor regression in
the exercised animals. In the (b) experiment, the weight of tumors
of the control animals in every instance was also larger than in the
animals given injections, and there were also cases of complete tumor
regression in injection group.
The authors [21] concluded that a tumoristatic factor may be
produced by muscle contraction and this tumoristatic effect of
exercise may not be due entirely to a divergence of energy from tumor
growth during exercise. They identified no decreased mitotic activity
of the tumors by histological examination. The study could not
explain the nature of the substance and the mechanism of its action
in retarding tumor growth [21].
Newton - 1965
Newton [22] believed the tumor growth development could be
modified by tumor-inhibitors and life experiences. The hypothesis
in this study was that social isolation and minimum physical activity
could contribute to fast tumor development. The experiment
considered how differential early treatment would compare and
interact with later exercise in affecting the response to implanted
tumor cells in rats. Newton [24]. Compared infantile-manipulated
(x) with non-manipulated groups (c). The manipulation was a
stressor defined as early treatment, which consisted of removing pups
from the nest by tweezer and placing them in a separate cardboard
container for 3 min. daily from 2-to-7 days of age.
Six groups were created Ax, Bx, Cx and Ac, Bc, and Cc. The A
groups were not submitted to exercise. The B groups were exercised
(through 138 hours of walking exercise over 10 days) post tumor
inoculation. The C groups exercised prior to inoculation (for 50 hours
over a period of 5 days) and after (138 hours of walking exercise over
10 days). All animals were inoculated subcutaneously in the right
flank at 50 days of age with a Walker 256 tumor. The results showed
that exercise retarded tumor growth. This was even more so when
it was combined with infantile manipulation. Overall mortality was
delayed only by manipulation, and was not postponed by exercise.
Manipulated rats exercised only after implantation presented with a
shorter survival time than those manipulated and exercised prior to
and following cell inoculation. Pre-implantation exercise apparently
conditioned manipulated animals against inflammation of exercise
that followed inoculation [22].
Figure 1
Discussion
An important further step in the field of ‘exercise and cancer’
addresses the translation from scientific findings into practice. Until
now, the optimal type, frequency, duration and intensity of exercise
training for cancer patients remain largely unknown [23]. In animal
models, physical exercise has been shown to suppress tumor initiation
and progression. The neurotransmitter dopamine is closely related to
movement and exhibits antitumor properties [24].
The primary hypotheses in experimental animal models revealed
the effects of exercise on tumor growth related to metabolic energetic
factors and stress. One possible interpretation of this is (a) exercise
retards tumor growth by using energy of tumor cells or secretion of
substance produced in muscular fatigue, and (b) exercise could be
tumor inhibitor or inductor agent, together with other additional
conditioners and factors related to life experiences within individuals.
Generally, the studies presented here showed decreased tumor
growth in animals submitted to exercise. Rusch and Kline21, Rashkis22
and Hoffman et al. [21] investigated exercise as an agent associated to
energetic metabolism of tumor cells. However, Newton [22] suggested
the existence of more factors modulating tumor growth, and factors
related to life experiences of individuals would also modulate tumor
growth.
Alterations to cellular metabolism should be considered a crucial
hallmark of cancer [25]. Tumor cells utilise increased glucose uptake
and lactate extrusion by tumors, and consequent pH decreases in
surrounding tissues, even in presence of ample oxygen – the Warburg
effect [26]. The Warburg effect has been demonstrated in different
types of tumors and the concomitant increase in glucose uptake
has been exploited clinically for the detection of tumors [27]. We
consider that the findings of Rusch and Kline [19] and Hoffman et al.
[21] studies can plausibly be justified based upon the Warburg Effect.
Steiner et al. [28] investigated whether voluntary physical
activity, initiated prior to the development of mammary tumors,
could attenuate tumor development and growth in mouse model of
breast cancer. Voluntary wheel running activity was more effective
in preventing progression of tumor growth as opposed to inhibiting
tumor initiation, independent of an energy imbalance, and possibly
because of minimized stress placed on the animal by voluntary exercise.
In another study, the same authors found reduced tumor volume and
reduction in plasma concentration of inflammatory substances in
mice post-exercise; an apparent benefit of exercise training on breast
cancer progression mediated by its anti-inflammatory potential [29].
Exercise is a stressor to the human body, and the magnitude of
this stress appears to be related to the volume and intensity of exercise
to which the individual is exposed [30]. According Justice [31], the
type of tumor and the time of stress application are crucial on tumor
induction. Several hypotheses have been generated to explain the
stress-cancer relationship: (a) stress-inhibition, stress administered
during tumor development slows tumor growth of non-viral origin;
(B) stress-recovery after the stress is stopped, the tumor grows more
rapidly than in animals not subjected to stress; (C) facilitating the
immune-, viral tumors grow faster during exposure to the stressor
due to immunosuppression; (D) immune-recovery after stress
stopping, there is a recovery of the immune system which inhibits the
development of viral tumors. Studies presented byJustice [31] using
PE as a stressor showed the amount of exercise imposed on rats and
effects in tumorigenesis can have a protective or tumor-inducing role.
If exercise results in significant muscle damage, inflammatory
processes can play an important role in production of free radicals,
oxidative stress inducers, and consequently DNA damage,
tumorigenesis, and increase mitosis of tumor cells [31]. The classical
theory of tumorigenesis involves a multistage process: (a) initiation
of the tumor, by consequences of the initial interaction of the tissues
with the carcinogen; (B) promotion of the tumor, by processes that
facilitate the expression of the phenotype started in the tissue; and
(c) tumor progression, in which there is proliferation and invasion of
other tissues, with changes in gene expression and DNA damage of
tumor cells [32].
Paradoxically, regular PE can be beneficial in preventing
tumorigenesis through changes in hormone levels, growth factors,
and reduction of obesity. Further, it may reduce pro-inflammatory
mediators and reduce chronic inflammation [32]. Thus, despite the
increased production of free radicals, regular PE and higher levels of
physical activity assist in balancing oxidative damage in DNA repair
[33]. The chronic effect of PE results in the neuroendocrine system
adaptations that may cause reduction in stress hormones responses,
and lead to reduced baseline levels of stress hormones [30]. Cook et
al. [34] found symptoms and exacerbated inflammatory responses
in male-mice placed on systematic PE forced to moderate intensity
and attenuated in those who practiced PE voluntarily. Jones et al. [17]
justify the training effect by aerobic PE on the reduction of tumor
development speed by increasing vascularity in the tumor cells,
"normalizing" the inside of the affected tissue. As hypoxia in tumor
tissues decreases due to the increased blood supply, fewer metastases
occur, so the tumor development is reduced [17].
The pioneering experiments reviewed with animals were reliant
upon unethical and unviable PE protocols for humans. There is a
clear difference between current and pioneer experiments. Currently,
several factors are considered and analyzed to explain the effects
of exercise on cancer, that are usually associated with the immune
system. Pioneering hypotheses considered isolated factors, as stress,
energy or substances only. The advances in science and technology
will ultimately allow greater depth of knowledge in this area, but
the preliminary advances have produced comparable findings in
considering pioneering work to recent studies. These include evidence
on the role of inflammatory markers of the immune system and body
responses to stress.
The pioneering hypotheses of experimental animal models (that
the effects of physical exercise have an inhibitory influence on tumor
growth) justified this relationship by energy metabolism. Currently,
studies show that PE can have protective or cancer-inducing effect,
depending on the intensity, duration, and type of exercise. The
mechanisms involved in PE effects on tumor growth are in an early
stage of developing understanding and remain unclear, possibly due
to the heterogeneous nature of tumors and biological intra-individual
variation. The view that there is a relationship between the amount of
PE and tumor grow will be the subject of further work.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016; 66: 7-30.
- Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol.2008; 8: 59-73.
- Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell. 2009; 136: 823-837.
- Spinola AV, Manzzo Id, RochaCd. As relações entre exercício físico e atividade física e o câncer. ConScientiae Saúde [Internet].2007; 6: 39-48.
- Courneya KS. Physical activity in cancer survivors: a field in motion. Psychooncology. 2009; 18: 337-342.
- Battaglini C, Bottaro M, Dennehy C, Rae L, Shields E, Kirk D, et al. The effects of an individualized exercise intervention on body composition in breast cancer patients undergoing treatment. Sao Paulo Med J. 2007; 125: 22-28.
- Campbell KL, Neil SE, Winters-Stone KM. Review of exercise studies in breast cancer survivors: attention to principles of exercise training. Br J Sports Med.2012; 46: 909-916.
- Craft LL, Vaniterson EH, Helenowski IB, Rademaker AW, Courneya KS. Exercise effects on depressive symptoms in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012; 21: 3-19.
- Demark-Wahnefried W, Jones LW. Promoting a healthy lifestyle among cancer survivors. Hematol Oncol Clin North Am.2008; 22: 319-342.
- Loprinzi PD, Cardinal BJ, Winters-Stone K, Smit E, Loprinzi CL. Physical activity and the risk of breast cancer recurrence: a literature review. Oncol Nurs Forum.2012; 39: 269-274.
- Saarto T, Sievanen H, Kellokumpu-Lehtinen P, Nikander R, Vehmanen L, Huovinen R, et al. Effect of supervised and home exercise training on bone mineral density among breast cancer patients. A 12-month randomised controlled trial. Osteoporos Int.2012; 23: 1601-1612.
- Salhi B, Demedts I, Simpelaere A, Decraene S, Vermaelen K, Surmont V, et al. Endurance and Resistance Training in Radically Treated Respiratory Cancer patients: A Pilot Study. Rehabil Res Pract. 2010: 2010; 481546.
- Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010; 42: 1409-1426.
- Wolff G, Toborek M. Targeting the therapeutic effects of exercise on redox-sensitive mechanisms in the vascular endothelium during tumor progression. IUBMB Life. 65: 565-571.
- Faustino-Rocha AI, Gama A, Oliveira PA, Alvarado A, Neuparth MJ, Ferreira R, et al. Effects of lifelong exercise training on mammary tumorigenesis induced by MNU in female Sprague-Dawley rats. Clin Exp Med. 2017; 17: 151-160.
- Faustino-Rocha AI, Silva A, Gabriel J, Gil da Costa RM, Moutinho M, Oliveira PA, et al. Long-term exercise training as a modulator of mammary cancer vascularization. Biomed Pharmacother.2016; 81: 273-280.
- Jones LW, Viglianti BL, Tashjian JA, Kothadia SM, Keir ST, Freedland SJ, et al. Effect of aerobic exercise on tumor physiology in an animal model of human breast cancer. J Appl Physiol.2010; 108: 343-348.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009; 62: e1-34.
- Rusch H, Kline B. The effect of exercise on the growth of a mouse tumor. Cancer Res. 1944; 4: 116-118.
- Rashkis H. Systemic stress as an inhibitor of experimental tumors in Swiss mice. Science. 1952; 116: 169-171.
- Hoffman SA, Paschkis KE, Debias DA, Cantarow A, Williams TL. The influence of exercise on the growth of transplanted rat tumors. Cancer Res.1962; 22: 597-599.
- Newton G. Tumor susceptibility in rats: role of infantile manipulation and later exercise. Psychol Rep. 1965; 16: 127-132.
- Wiskemann J, Scharhag-Rosenberger F. The evolving role of exercise in cancer patients: recent developments, recommendations and future directions 2016. Future Oncol.2016; 12: 1541-1544.
- Zhang QB, Zhang BH, Zhang KZ, Meng XT, Jia QA, Bu Y, et al. Moderate swimming suppressed the growth and metastasis of the transplanted liver cancer in mice model: with reference to nervous system. Oncogene. 2016; 35: 4122-4131.
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nature Reviews Cancer.2011; 11:85-95.
- Ferreira LM. Cancer metabolism: the Warburg effect today. Exp Mol Pathol.2010; 89: 372-380.
- Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008; 134: 703-707.
- Steiner JL, Davis JM, McClellan JL, Enos RT, Murphy EA. Effects of voluntary exercise on tumorigenesis in the C3(1)/SV40Tag transgenic mouse model of breast cancer. Int J Oncol.2013; 42: 1466-1472.
- Murphy EA, Davis JM, Barrilleaux TL, McClellan JL, Steiner JL, Carmichael MD, et al. Benefits of exercise training on breast cancer progression and inflammation in C3(1)SV40Tag mice. Cytokine.2011; 55: 274-279.
- Hackney AC. Exercise as a stressor to the human neuroendocrine system. Medicina (Kaunas).2006; 42: 788-797.
- Justice A. Review of the effects of stress on cancer in laboratory animals: importance of time of stress application and type of tumor. Psychol Bull.1985; 98: 108-138.
- Kavazis AN, Powers SK. Impact of Exercise, Reactive Oxygen and Reactive Nitrogen Species on Tumor Growth. In Exercise, Energy Balance, and Cancer. Springer. 2013. pp. 7-20.
- McCullough LE, Santella RM, Cleveland RJ, Millikan RC, Olshan AF, North KE, et al. Polymorphisms in DNA repair genes, recreational physical activity and breast cancer risk. Int J Cancer.2014; 134: 654-663.
- Cook MD, Martin SA, Williams C, WhitlockK, Wallig MA, PenceBD, et al. Forced treadmill exercise training exacerbates inflammation and causes mortality while voluntary wheel training is protective in a mouse model of colitis. Brain Behav Immun.2013; 33: 46-56.