Research Article
Cognitive Profiles in Children Treated for Brain Malignancies as Compared to Children with Traumatic Brain Injuries
Ingrid van’t Hooft1* and Annika Lindahl Norberg2
1Department of Neuropediatric, Astrid Lindgren´s Children´s Hospital, Karolinska University Hospital, Stockholm, Sweden
2Department of Women’s and Children’s Health, Karolinska Institutet, Stockholm, Sweden
*Corresponding author: Ingrid van’t Hooft, Department of Neuropediatric, Astrid Lindgren’s Children’s Hospital, Karolinska Hospital, SE-17176 Stockholm, Sweden
Published: 15 May, 2017
Cite this article as: van’t Hooft I, Norberg AL. Cognitive
Profiles in Children Treated for Brain
Malignancies as Compared to Children
with Traumatic Brain Injuries. Clin
Oncol. 2017; 2: 1291.
Abstract
Objective: We investigated the cognitive profiles of children treated for brain tumours as compared
to children treated for brain traumas. Furthermore how various moderators such as age at the time
of injury/diagnosis, time since injury/ diagnosis, and severity of the injury might correlate with
outcome.
Methods: 64 children treated for brain tumours and 77 with traumatic brain injures (6-18 yrs) who
were all assessed with cognitive tests between 2000 and 2009 were investigated.
Results: There were no significant difference between the two diagnostic groups on total IQ, verbal
comprehension, perceptual organization, processing speed and freedom of distractibility or verbal
working memory. Moreover, no significant difference between, the children with brain tumours,
who had undergone cranial radiation therapy and those who had not.
Conclusions: Findings indicate that all children who have been treated for brain tumours
independent of treatment should be screened with cognitive measures and followed over time.
Keywords: Children; Cognitive profiles; Traumatic brain injury; Brain malignancies
Introduction
Paediatric oncology has during the last decades been very successful in treating childhood cancer. Neurosurgery, in combination with Cranial Radiotherapy (CRT) and or chemotherapy, is the treatment modalities that are available for children with malignant brain tumours. However, this treatment is not free from complications. Common medical problems are neurological [1], endocrinological [2] or oncological [3]. Moreover there is also a significant risk of neuro-cognitive sequelae [4]. This risk is highest for those children that have got both CRT and chemotherapy although children that just have been operated or just have got chemotherapy seem also at risk [5,6]. Deficits in cognitive processing skills are also commonly observed in the long-term follow-up of children with other aetiologies to the acquired brain injury. Deficits in attention, memory, executive functions and speed of information processing are reported to be the most common cognitive problems [7]. If these cognitive deficits are present during development they impede learning and the acquisition of new skills and knowledge, resulting in the global cognitive dysfunction commonly observed in the long-term follow-up of children with acquired brain injuries [8]. Clinical evaluations and observations of the cognitive consequences after acquired brain injuries in childhood indicate that there may be differences in cognitive consequences depending on the character of the injury. In order to develop specific interventions for children with neuro-cognitive consequences after acquired brain injuries it seems crucial to investigate these possible differences in cognitive style dependant on the aetiology of the injury. The aim of the present study was therefore to investigate and compare the cognitive profiles in children treated for brain tumours as compared to children with a Traumatic Brain Injury (TBI).
Materials and Methods
The paper presents an investigation of a clinical sample, and is based on historical data from
neurocognitive examinations during ten years in a Neuropediatric unit at a children´s hospital in
Sweden.
Participants
The sample included children treated for malignant brain tumours
and children with TBI, who were followed at the Neuropediatric
and the Oncology Units at Astrid Lindgren Children’s Hospital
Stockholm during the period 2000 - 2009. The criterion for inclusion
was an investigation with cognitive tests including a complete WISC
(Wechsler Intelligence Scales for Children) protocol. Annually, about
20-25 children are diagnosed with brain tumours at Astrid Lindgren
Children’s Hospital in the Stockholm region and 120 children are
yearly referred to the Neuropediatric rehabilitation unit after TBI. In
2006 the hospital developed a long term neurorehabilitation program
where approximately 75% of the children with brain tumours are
referred to neuro-cognitive examination after the cancer treatment
has been completed. About 50 children/year with TBI are referred to
neuro-cognitive investigation. Before the year 2006 children both with
brain tumours as well as TBI were investigated to a lesser extent. The
neuro-cognitive investigation normally contains the WISC for testing
general cognitive level of children of 7-18 years of age. However,
complete WISC protocols are not available for children with too poor
functions or those who were not cooperating, i.e. those who could not
or would not complete the test. In total, data from 66 children who
had been treated for brain tumours, and 74 children with traumatic
brain injuries were included in the study. Descriptive data for the two
groups are presented in Table 1. The brain tumour group involved
patients diagnosed between 1992 and 2007. The children with TBI
were injured between 1993 and 2008.
Assessments
Cognitive functions: The general intellectual ability of the
children was assessed employing the Wechsler Intelligence Scales
for Children (WISC-III, WISC IV), with a mean of 100 and SD of
15 [9]. Factor analyses were estimated from the WISC-III/WISC IV
on Verbal Comprehension, Perceptual Organization, Freedom of
Distractibility (attention), and Speed of Processing.
Memory: In the 15 word test [10], assessing verbal memory, the
children had to listen to simple unrelated words presented 5 times. The
task was after each presentation to repeat as many words as possible
by free recall. In addition, the total number of words remembered
in connection with delayed free recall 40 minutes later was scored
separately. In accordance with the Rey-Osterrieth Complex Figure Recall [10], which is a measure of visuo-spatial memory, the children
were asked to reproduce a complex geometrical design 30 minutes
after having copied it and without knowing in advance that they
would be requested to do this. The accuracy score is the number
of elements remembered and reflects the amount of information
retained for this period of time.
Severity of the brain injury: A rough estimation of the potential
severity of the brain injury, with respect to possible neuro-cognitive
deficits, was obtained. Considering the adverse effect of radiation
against the developing brain, children who had received CRT as part
of their brain tumour treatment were assigned to the severe injury
group. According to the approach generally used for estimating the
severity of TBI, children with a Glascow Coma Scale (GCS) score of
< 8 at initial arrival to the hospital were considered having a severe
injury.
Procedure
Data for the most recent test performance of each child were
collected from the medical records, together with medical and
demographic data. The study was approved by the Ethical Committee
at Karolinska Institutet.
Statistical analyses
In order to analyze the differences in neuro-cognitive test scores
between brain tumour survivors and children with TBI at a group
level, t-test were performed. Moreover, post-hoc ANOVA was used
to examine any differences in test scores between all four sub groups:
the children with more or with less severe injury due to brain tumour
or TBI, respectively. Bonferroni correction was applied to avoid type-
1 errors due to multiple tests in the ANOVA. Chi2 calculations were
used to compare brain tumour survivors and children with TBI as
regards the frequency of children performing significantly below
average for age. Similarly, the frequency of scores significantly below
average for age was analyzed in relation to severity of the brain injury
using Chi2 tests. Finally, the association between cognitive outcome
and the variables age at the time of injury, and time since injury/cancer diagnosis was analysed with Pearson correlation, 2-tailed.
Table 1
Table 2
Table 2
Mean, standard deviation and range for neuro-cognitive tests of children with brain tumours and children with TBI.
Figure 1
Results
Descriptive statistics
The two study groups differed significantly with respect to age
at the time of injury/tumour diagnosis, with the children with TBI
typically being older than the children who were diagnosed with a
brain tumour (t=‑7.8, p < .001; Table 1). Moreover, the time elapsed
from diagnosis/injury to the neurocognitive testing was significantly
longer in the oncology group (t=5.1, p < .001). Consequently, the
child’s age at testing differed in the same direction (t=5.1, p < .001).
Brain tumour and TBI: Neurocognitive outcome
No statistically significant differences were found in
neurocognitive test outcomes between brain tumour survivors and
children with TBI (Table 2).
Difference from average for age
At a group level, the scores for full scale IQ as well as the four
indices of WISC were slightly below average for age for both brain
tumour survivors and children with TBI, with the Processing speed
factor demonstrating the largest difference from average for age for
both groups. Subsequently, the frequency of test results significantly
below the average for age was examined. Forty four per cent of the
children who had been treated for brain tumours, and 34% of the
children with TBI had a Full scale IQ below average, indicating
general neurocognitive difficulties.
Severity of the brain injury: Neurocognitive outcome
Subsequently analyses were made of the severity groups of brain
tumour (radiation therapy or no radiation therapy) and TBI (GCS
<8 or >/=8 at the initial arrival at the hospital). Test outcomes are
presented in Table 3. Comparisons of the WISC full scale IQ and
indices and the 15 Words verbal memory between all four severity
groups rendered a few statistically significant differences (post-hoc
ANOVA with Bonferroni correction): the children with GCS <8 at
arrival had lower scores than those with GCS >/=8 regarding full
scale IQ (mean difference=12.8, p < 0.05) and perceptual organisation
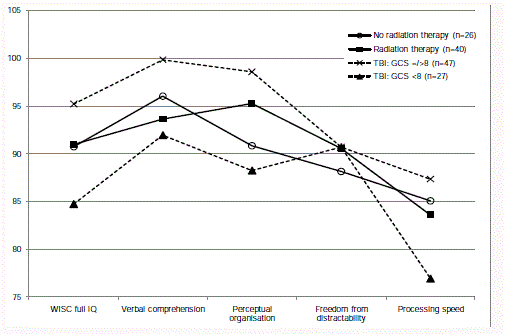
(mean difference=12.8, p < 0.05). Moreover, the four severity groups
displayed fairly similar profiles, with processing speed as the main
difficulty (Figure 1).
Difference from average for age
The frequencies of radiated and non-radiated brain tumour survivors with markedly poor neuro-cognitive outcome (test scores
significantly lower than average for age) are presented in Table 3. A
further exploration of the neurocognitive outcome of children who
had or had not received radiation revealed no statistically significant
differences. In addition to WISC scores, verbal and visuo-spatial
memory outcome is presented (Table 3).
Time since injury/brain tumour diagnosis
Time elapsed since diagnosis of the brain tumour was correlated
to WISC Full scale IQ (r=-.36, p < .01) and the indices Verbal
comprehension (r=-.35, p < .05) and Processing speed (r=-.35, p
< .05), indicating a poorer performance in children a longer time since
diagnosis. Among the children with TBI, there were no associations
between time elapsed since the head trauma and neuro-cognitive
outcome.
Age at injury
The child’s age at brain tumour diagnosis or head trauma,
respectively, was not systematically associated with test scores
(Pearson’s correlation).
Table 3
Table 3
Brain tumour survivors with neurocognitive outcome significantly lower
than average for age, in relation to cranial radiation therapy (CRT).
Discussion
The comparison of neuro-cognitive profiles between children who
had been treated for brain tumours and children with TBI revealed
no significant differences. Also when the groups were divided into
severity groups no statistical difference was found between children
surviving brain tumour and children with TBI. However, children
treated with CRT and children with GCS < 8 showed cognitive results
with a group mean more than one standard deviation below average
for age indicating general neurocognitive difficulties with slow speed
of processing and attentional difficulties as the most noticeable
dysfunctions. These data supports earlier findings [11]. The essential
finding in the present study was instead that no significant difference
was found between children with brain tumours treated with CRT
and those who were not. This applied to cognitive level, verbal
comprehension factor, perceptual organization, processing speed,
and freedom of distractibility as well as verbal and visuospatial
memory. Both groups showed results below average for age on all
these measures. This contrasts previous research suggesting that the most adverse effects are to be seen in patients who have undergone
CRT [5]. These deficits have mainly been attributed to the effects of
CRT, but there is increasing recognition that tumour infiltration,
surgical resection and chemotherapy likely play a role in the cognitive
outcome of brain tumour patients [12]. The present results indicate
that surviving a brain tumour, independently of treatment, implies a
risk of overall decline in intellectual functioning with processing speed
and attentional factors being most affected. The children in the brain
tumour group indicated a decline in neurocognitive performance
the longer time had passed since diagnosis. This cognitive decline is
earlier reported in the literature and indicates that aside from declines
in intellectual and academic achievements a sequence of deterioration
is found beginning less than 6 months following treatment. This
consists of 1) initial deterioration in memory and attention skills
2) then perceptual disturbances of visual spatial organization and
sequencing of actions, 3) a decline in analysis, synthesis and abstract
thought and eventually 4) a decreased vocabulary [11]. The child’s
age at brain tumour diagnosis or head trauma, respectively, was
in this retrospective study not systematically associated with test
scores, possibly due to small groups and heterogeneity. This result
is in contrast to several studies, where young age at injury has been
found to negatively influence outcome following traumatic brain
injury [13], as well as children treated for brain tumours [5,14]. The
clinical characteristic of the present study sample is a limitation that
prevents us from drawing reliable conclusions. We have reason to
suspect that the children were not systematically recruited to the
sample. For example, all children who had undergone CRT at the
hospital are referred to neuro-cognitive follow-up. On the other
hand, of the non-radiated children only those with obvious problems
used to be referred. Yet, it can be argued that less obvious problems
may be overseen among these children, if we remain focusing on
children with cranial radiation only. Preventing these late effects on
cognitive processing is a challenge for both the medical team and
for psychologists and rehabilitation specialists. Prevention depends
in part on the ability to predict those at greatest risk. Advances in
neuroimaging hold promise for helping to predict during the acute
phase of treatment those patients who may suffer the greatest neurocognitive
declines. Consequently these patients may have risk-adapted
therapy that seeks to lessen morbidity. For survivors who show neurocognitive
decline following cancer treatment, rehabilitation similar to
that used for children with TBI have shown some effectiveness Mean, standard deviation and range for neuro-cognitive tests of children with brain tumours and children with TBI.[15-17]. For newly diagnosed patients who are identified as being at risk
prophylactic interventions such as cognitive training may offer the
best hope of preventing late effects. There is a great need for long term
follow-up programs for children treated for brain tumours. These
follow up programs ought to include not only medical aspects but
also neuro-cognitive and psychological late effects. In order to be able
to recommend treatment and pedagogic interventions we suggest a
model where all children who have been treated for brain tumours
independent of treatment at an early stage should be screened with
cognitive measures and at key ages followed over time.
In conclusion, in spite of the methodological shortcomings of
the study, the findings are intriguing and encourage well designed
prospective studies in larger groups appear to be required in order to
assess possible differences in cognitive deficits between children with
different aetiologies to an acquired brain injury.
References
- Lannering B, Sandström PE, Holm S, Lundgren J, Pfeifer S, Samuelsson U, et al. Classification, incidence and survival analyses of children with CNS tumours diagnosed in Sweden 1984-2005. Acta Paediatr. 2009; 98(10): 1620-1627.
- Packer RJ, Gurney JG, Punyko JA, Donaldson SS, Inskip PD, Stovall M, et al. Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: childhood cancer survivor study. J Clin Oncol. 2003; 21: 3255-3261.
- Spoudeas HA, Charmandari E, Brook CG. Hypothalamo-pituitary-adrenal axis integrity after cranial irradiation for childhood posterior fossa tumours. Med Pediatr Oncol. 2003; 40: 224-229.
- Armstrong GT, Liu Q, Yasui Y, Huang S, Ness KK, Leisenring W, et al. Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009; 101: 946-958.
- Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004; 5(7): 399-408.
- Sonderkaer S, Schmiegelow M, Carstensen H, Nielsen LB, Muller J, Schmiegelow K. Long-term neurological outcome of childhood brain tumors treated by surgery only. J Clin Oncol. 2003; 21: 1347-1351.
- Beers SR. Cognitive effects of mild head injury in children and adolescents. Neuropsychol Rev. 1992; 3(4): 281-320.
- Catroppa C, Anderson VA, Morse SA, Haritou F, Rosenfeld JV. Outcome and predictors of functional recovery 5 years following pediatric traumatic brain injury (TBI). J Pediatr Psychol. 2008; 33: 707-718.
- Wechsler D. Wechsler Intelligence Scale for Children. 4 ed. San Antonio, TX: Harcourt Assessment Inc. 2003.
- Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4 ed. New York: Oxford University Press. 2004.
- Briere ME, Scott JG, McNall-Knapp RY, Adams RL. Cognitive outcome in pediatric brain tumor survivors: delayed attention deficit at long-term follow-up. Pediatr Blood Cancer. 2008; 50: 337-340.
- Askins MA, Moore BD 3rd. Preventing neurocognitive late effects in childhood cancer survivors. J Child Neurol. 2008; 23(10): 1160-1171.
- Anderson V, Jacobs R, Spencer-Smith M, Coleman L, Anderson P, Williams J, et al. Does early age at brain insult predict worse outcome? Neuropsychological implications. J Pediatr Psychol. 2010; 35: 716-727.
- Palmer SL. Neurodevelopmental impact on children treated for medulloblastoma: a review and proposed conceptual model. Dev Disabil Res Rev. 2008; 14(3): 203-210.
- van't Hooft I, Lindahl Norberg A. SMART cognitive training combined with a parental coaching programme for three children treated for medulloblastoma. Neuro Rehabilitation. 2010; 26: 105-113.
- van 't Hooft I, Andersson K, Bergman B, Sejersen T, von Wendt L, Bartfai A. Sustained favorable effects of cognitive training in children with acquired brain injuries. NeuroRehabilitation. 2007; 22(2): 109-116.
- Butler RW, Haser JK. Neurocognitive effects of treatment for childhood cancer. Ment Retard Dev Disabil Res Rev. 2006; 12(3): 184-191.