Review Article
Radiologic-Pathologic Correlation in Lung Cancer Presenting as a Subsolid Nodule: Room for Improvement?
Annemie Snoeckx1*, Pieter Reyntiens1, Patrick Pauwels2, Paul E. Van Schil3, Maarten J.
Spinhoven1, Paul M. Parizel1 and Jan P. van Meerbeeck4
1Department of Radiology, Antwerp University Hospital and University of Antwerp, Wilrijkstraat, Belgium
2Department of Pathology, Antwerp University Hospital and University of Antwerp, Wilrijkstraat, Belgium
3Department of Thoracic and Vascular Surgery, Antwerp University Hospital and University of Antwerp, Wilrijkstraat, Belgium
4Department of Thoracic Oncology, Antwerp University Hospital and University of Antwerp, Wilrijkstraat, Belgium
*Corresponding author: Annemie Snoeckx, Department of Radiology, Antwerp University Hospital and University of Antwerp, Wilrijkstraat 10, 2650 Edegem, Belgium
Published: 15 May, 2017
Cite this article as: Snoeckx A, Reyntiens P, Pauwels P,
Van Schil PE, Spinhoven MJ, Parizel
PM, et al. Radiologic-Pathologic
Correlation in Lung Cancer Presenting
as a Subsolid Nodule: Room for
Improvement?. Clin Oncol. 2017; 2:
1290.
Abstract
Pulmonary nodules are a common finding on Computed Tomography (CT) imaging studies. Nodules are becoming more frequently encountered in daily practice due to widespread use of CT and increasing interest in lung cancer screening by low dose CT. In 2011, the term bronchioloalveolar carcinoma was abandoned and the new IASLC/ATS/ERS classification system of lung adenocarcinoma and its precursors was introduced. In 2015, this new classification system was adopted by the World Health Organization (WHO). In this new classification findings on histopathology are correlated with imaging studies. This correlation holds imperfections, leaving room for improvement. A correct classification of pulmonary nodules into solid or subsolid is key to precise nodule management. Furthermore, morphological assessment of subsolid nodules is mandatory and essential for follow-up and assessing likelihood of invasiveness. A significant group of lesions that are pure ground glass without a solid component and hence suspicious for Atypical Adenomatous Hyperplasia (AAH) or Adenocarcinoma In Situ (AIS) on imaging grounds, turn out to be invasive adenocarcinoma. Other lesions that are part-solid on CT and suspicious for invasive adenocarcinoma turn out to be only AIS at resection. Moreover, nodule classification and morphological assessment are prone to variability among radiologists. Computer aided techniques and quantitative CT-analysis are on the rise. These techniques will create room for standardization and will make prospective studies regarding radiologic-pathologic correlation in subsolid nodules more precise and reliable. More accurate radiologic-pathologic correlation will lower the risk of over-/underdiagnosis and will aid in optimal patient selection for surgical treatment.
Introduction
Pulmonary nodules are a common finding on Computed Tomography (CT) imaging studies
of the chest, with adenocarcinoma being the most frequent encountered histological subtype of
lung cancer presenting as pulmonary nodule [1,2]. Pulmonary adenocarcinomas consisted of a large
and heterogeneous group of tumors with different types of histological growth patterns. In 2011,
the term Bronchiolo-Alveolar Carcinoma (BAC) was abandoned and a new lung adenocarcinoma
classification was published by the International Association for the Study of Lung Cancer (IASLC),
the American Thoracic Society (ATS) and the European Respiratory Society (ERS) [3]. This new
classification was officially adopted by the World Health Organization (WHO) in 2015 [4]. Although
this new classification was mainly based on histological criteria, it was developed in collaboration
with clinical, molecular and surgical colleagues as well as radiologists. In this new system, findings
on histology were correlated with CT-imaging criteria.
Ground Glass Nodules (GGN) appear on thin section CT-images as hazy increased opacities
of lung, with preservation of bronchial and vascular margins. Ground-glass is less opaque than
consolidation in which bronchovascular margins are obscured [5]. On histological specimens,
this correlates with a lepidic growth pattern, a pattern that is defined as tumor cells proliferating
along the surface of intact alveolar walls without stromal or vascular invasion [6]. Lesions that only
consist of ground glass are referred to as “pure GGN”. Lesions that include a combination of both
ground-glass and solid components (with obscuring the lung architecture) are referred to as “partsolid
GGNs”. Both pure GGNs and part-solid GGNs are considered and categorized as “subsolid” nodules [5].
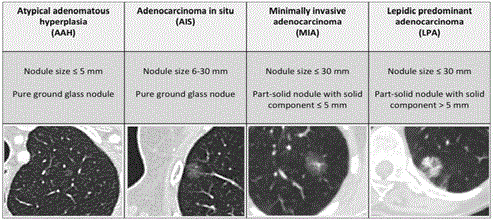
The 2011, IASLC/ATS/ERS classification defines 4 types of lesions
in the adenocarcinoma spectrum in relation to subsolid nodules with
a maximum diameter of 3cm: Atypical Adenomatous Hyperplasia
(AAH), Adenocarcinoma In Situ (AIS), Minimally Invasive
Adenocarcinoma (MIA) and Lepidic Predominant Adenocarcinoma
(LPA). Each of these adenocarcinoma subtypes has a CT-image
correlate (Figure 1) AAH and AIS are believed to be pre-invasive
lesions for lung adenocarcinoma, presenting on CT as pure ground
glass lesions. AAH is usually smaller than 5mm and AIS larger than
5mm on any view of the CT image. Both AAH and AIS show no solid
component on thin-section CT. AIS can be discretely more opaque
than AAH. Neither AAH nor AIS are invasive on histopathology.
Any pure ground glass lesion larger than 3cm is considered to be LPA.
MIA represents a lesion smaller than 3cm with a solid component
(on imaging) and invasive component (on histopathology) of 5mm
or smaller. LPA is a subsolid lesion that is also not larger than 3cm
with a solid or invasive component of more than 5mm [3,4].
Nowadays, ground glass and part-solid nodules are more
frequently encountered in daily practice than years ago. It is unclear
if this is only a perception or that this represents a real increase in
incidence. The widespread use of CT in clinical practice and the use
of multidectector CT-scanners with finer CT collimation certainly are
important factors for the increase in detection of subsolid nodules.
Furthermore, numerous large lung cancer screening trials have been
initiated in the past 10 to 15 years. These trials have given more insight
in the incidence, evolution and prognosis of these adenocarcinoma
precursors. In the Early Lung Cancer Action Project (ELCAP), 2892
part-solid lesions were found among 5% of 57,496 participants [7]. In
the National Lung Cancer Screening Trial (NLST), which is the largest
randomized lung cancer screening trial, 9.4% of 26,722 participants
presented with one or more subsolid nodules [8]. In the first round
of the large Dutch-Belgian Randomized Lung Cancer Screening
Trial (NELSON), 2.0% of the total of 8673 nodules found in 7557
participants were subsolid nodules (both pure ground glass and partsolid)
[9]. Although the incidence of subsolid nodules is significantly
lower than that of solid nodules and masses, these lesions cannot be
ignored and pose future challenges.
A correct classification of pulmonary nodules is important since
the likelihood of malignancy is larger in subsolid nodules compared
to solid nodules [10]. Since subsolid nodules have a different
prognosis than solid nodules, they require a different management
approach. Moreover, invasive adenocarcinomas are more aggressive
than adenocarcinoma precursor lesions [8,11]. The size (or absence)
of a solid component on CT is correlated with the prevalence of an
invasive component and is therefore crucial for clinical decision
guidance. Furthermore survival in subsolid lesions depends on the
invasive (or solid) component and not on the lepidic (or ground
glass) component [12-16]. For this reason in the 8th edition of the
TNM classification only the solid part is considered to measure
the size of a lesion and determine the T descriptor [17]. The largest
unidimensional size is measured using the lung window setting on
chest CT scan.
Current guidelines for management and follow-up of subsolid
lesions are fully based on CT-imaging criteria. When assessing the
morphology of subsolid nodules, it is important to characterize these
lesions on contiguous thin sections (preferably 1.0mm or less than
1.5mm) and to evaluate the evolution comparing the lesions with
the oldest images available. In routine practice, the morphology of
lesions is visually assessed by a radiologist and measurement of both
the solid component and the lesion size is mainly done by manual
electronic caliper measurement. The border of the whole lesion as
well as the border between the solid component and ground glass
component is often hazy, making measuring a difficult task and
making reproducible measurements inaccurate.
To deal with the lack of detailed consideration of subsolid
lung nodules in the 2005 Fleischner guidelines [18], specific
recommendations for the management of subsolid pulmonary
nodules detected at CT, were published in 2013 [19]. These guidelines
were updated for both solid and subsolid nodules in 2017 [20]. The
British Thoracic Society also incorporated specific recommendations
for subsolid nodules in its guidelines for the investigations and
management of pulmonary nodules, published in 2015 [21].
Furthermore, the 8th edition of the TNM staging classification of
lung cancer, provides new criteria for cases presenting as multiple
nodules with ground glass or lepidic features [22]. This new staging
classification is based on CT-imaging findings of subsolid nodules.
To address the need of a dedicated classification system for lung
nodules in the era of lung cancer screening, the American College
of Radiologists (ACR) proposed the Lung-RADSTM or lung imaging
reporting and data system [23]. Subsolid nodules are incorporated in
this management and follow-up scheme, which is widely used in the
United States where – in contrast to Europe- lung cancer screening is
already embedded in daily practice.
All these guidelines and criteria are based on the theoretical
radiologic-pathologic correlation of this adenocarcinoma sequence,
having a major impact on management of subsolid nodules.
Six years after the publication of the new IASLC/ATS/ERS
classification guidelines, this new classification and the radiologicpathologic
correlation have certainly found their way into routine
clinical practice. Numerous issues however remain of which some
will be addressed in this article.
Figure 1
Theoretical Model
As discussed, there is a general correlation between imaging
appearance and histopathologic diagnosis. This model however is
theoretical, imperfect and not prospectively validated. Substantial
overlaps of imaging features of AAH, AIS, MIA and invasive lung
adenocarcinoma exist. To illustrate, we present 3 cases from a nonscreening
setting where the radiologic-pathologic correlation was not
accurate and adenocarcinoma subtype on pathology was different
than expected on imaging.
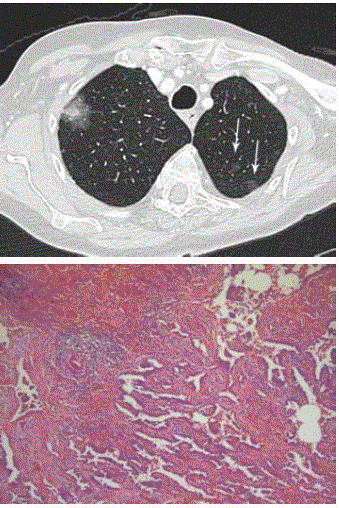
Case 1: An 82-year-old woman presented with a non-productive
cough for more than 1 year. Chest CT (Figure 2) showed a subsolid
nodule in the right upper lobe with a 17mm area of ground glass
(with relatively high attenuation) and solid component of 14mm.
Also note the small pure ground glass lesions in the left upper lobe
(white arrows). The size and morphology of the subsolid lesion was
suspicious for diagnosis of invasive adenocarinoma with lepidic
component. The histopathology specimen after wedge excision
showed findings consistent with minimally invasive adenocarcinoma.
Both lesions in the left upper lobe correlate on imaging with AAH
and AIS since they are pure ground glass nodules. Long term followup
of these lesions is foreseen.
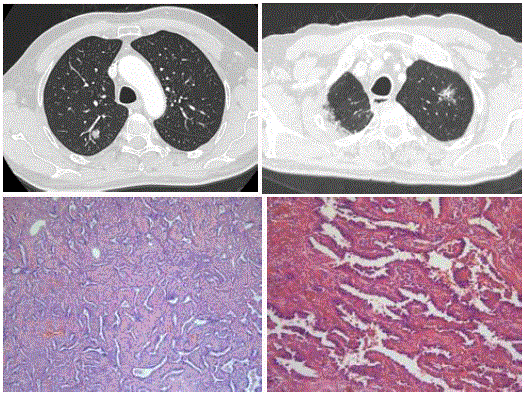
Case 2: A 70-year old man with previous surgery for a 1.3cm welldifferentiated
invasive adenocarcinoma (Figure 3A) in the right upper
lobe presented during follow-up with a persistent subsolid lesion in
the left upper lobe. CT examination 4 years after the initial surgery
showed a 22mm large subsolid lesions with 18mm solid component
and surrounding ground glass aspect (Figure 3B) in the left upper
lobe. The imaging appearance of this new persistent subsolid lesion
was suspicious for an invasive adenocarcinoma with lepidic growth.
Histopathology after complete resection of the nodule could not
reveal any invasive focus and diagnosis of Adenocarcinoma In Situ
(AIS) was made.
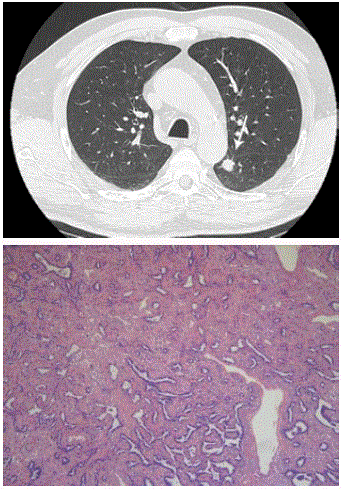
Case 3: In a 63-year-old man in whom a chest CT was performed
for persistent cough, a solid nodule (Figure 4) in the apex of the
left lower lobe with minor lobulation and no clear spiculated
morphology was discovered. Although this lesion did not show
any 18F-Fluorodexocyglucose (FDG) uptake on Positron Emission
Tomography (PET), the multidisciplinary tumor board found it
suspicious for primary lung cancer, mainly because of the pleural
indentation (white arrow). Although the lesion had a complete solid
appearance with absolutely no areas of ground glass, diagnosis of
predominant lepidic adenocarcinoma was made after lobectomy.
Numerous studies have looked into the Radiologic-Pathologic
Correlation (REF). Some of them correlate well with this model,
others do not. In a group of 300 lesions, Honda “et al”. [24] found
no invasive adenocarcinomas in the group of air-containing type
nodules (nodules with AIS or MIA morphology on imaging):
20.8% of AIS lesions on pathology had a solid component on CT.
In a retrospective study, 5mm was the highest solid component
size threshold with a sensitivity of 100% [23]. This means that in
patients undergoing resection for a ‘suspicious’ subsolid nodule, a
solid component of less than 5mm enabled to rule out the presence of invasive adenocarcinomas [25]. A retrospective study of surgically
resected lesions showed in a white (non-Asian) population that in
the group of pure ground glass lesions, 38.4% of lesions were invasive
adenocarcinoma [24]. In the group of part-solid nodules (with
significant solid component) 6.7% of lesions were adenocarcinoma
in situ and 10.0% minimally invasive adenocarcinoma. In the group
of pure solid lesions, 7.1% of lesions were in situ adenocarcinoma en
42.9% of lesions minimally invasive adenocarcinoma [26]. Eguchi
“et al”. [27] retrospectively investigated 101 pure ground glass
lesions and found half of cases exhibited a pathological invasive
area. Moreover a quarter of resected pure GGNs were diagnosed
as invasive adenocarcinomas. These lesions were larger in size (>11
mm with sensitivity of 95.8%) and in general had an higher overall
density. A small study by Lee “et al”. [28] showed that MIA appeared
as pure GGNs in one third of cases (5 out of 15) and presented as
part-solid GGNs (with small central solid component) in two thirds
of cases (10 out of 15).
So, a significant group of lesions that are pure ground glass
without solid component, turn out to be invasive adenocarcinoma
and some subsolid lesions turn out to be AIS and show no invasive
focus.
Figure 2
Figure 2
Axial CT in lung window setting (A) shows subsolid lesions in the
upper lobes of both lungs. Note the large subsolid lesion in the right upper
lobe and numerous pure ground glass nodules (white arrow) in the left upper
lobe. Photomicrograph (B) shows predominantly adenocarcinoma in situ.
However, fibrotic areas with individual acinar structures and a desmoplastic
reaction suggest invasion (H-E stain; original magnification, x 20).
Figure 3
Figure 3
Axial CT in lung window setting (A) shows a solid nodule with
discrete bubble like lucencies in the right upper lobe. Chest CT examination
4 years later shows a new but persistent subsolid nodule (B) in the left upper
lobe. Photomicrograph (C,D) shows adenocarcinoma in situ with neoplastic
cells that appear monotoneous, growing along intact alveolar septa. The
nuclei are enlarged with mild cytologic atypica. The cells show nuclear
overlapping. (H-E stain; original magnification, x 20 (C), x 40 (D)).
Figure 4
Figure 4
Axial CT in lung window setting (Figure 4A) shows a solid nodule
in the apex of the left lower lobe. The nodule shows minor lobulation but
no spiculation. The nodule is purely solid without signs of ground glass
component. Photomicrograph (B) shows a predominant lepidic pattern (right
part of the image) but areas of acinar growth are also present (left part of the
image) (H-E stain; original magnification, x 20).
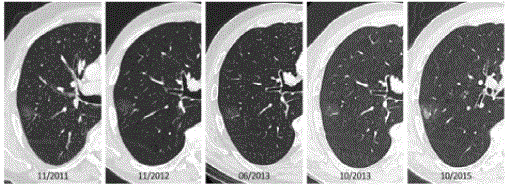
Figure 5
Figure 5
Four year follow-up in a 72-year-old man with a previous history of an oropharyngeal squamous cell carcinoma and lobectomy for 0.8cm invasive
adenocarcinoma in the left upper lobe. Follow-up chest CT’s show a pure ground glass lesion in the right upper lobe. Over a period of 3 years, the lesion did not
change morphologically. After 4 years, suddenly the ground glass component enlarged and a solid component of 15mm was noted. Histopathology after resection
showed a 21mm well-differentiated adenocarcinoma with areas of lepidic growth.
Lack in Observer Agreement for Classifying Nodules
As mentioned, guidelines are based on morphologic criteria
on CT. Once a nodule (solid or subsolid) is categorized, different
guidelines and follow-up schemes apply. As nodule management
is based on a visual assessment, how accurate is the radiologist
in defining the morphology of a nodule? The factors that affected
inter-and intra-observer agreement regarding the classification of
pulmonary nodules on low-dose CT images were analyzed [27] in
a retrospective series. A moderate overall interobserver agreement
(mean κ, 0.51) to categorize nodules into solid, part solid with a
solid component 5mm or larger of less than 5mm, and pure ground
glass, was reported. Disagreement was mainly related to either the
presence of a solid component in part-solid nodules or the size of this
solid component relative to the 5mm threshold, which are all crucial
criteria in different management protocols [29]. A retrospective
study on NLST-data by Singh “et al”. [30] also showed moderate to
substantial reader agreement on nodule growth and screening result,
and low reader agreement for changes in attenuation and margins.
Penn “et al”. [31] investigated the inter-reader variability of applying
the Fleischner guidelines for potential subsolid lung nodules. They
found only moderate inter-reader variability in particular regarding
the fit of subsolid nodule criteria and whether a solid component was
present. It may seem evident that expertise is important, but even
among experienced thoracic radiologists, inter-and intra-observer
agreement in differentiating solid from subsolid nodules on CT is
variable [32].
Pathology is believed to be the ‘golden standard’, but if
observer variability applies for radiologists, the same is true for
pathologists. Thunnissen “et al”. [33] provided strong evidence that
in adenocarcinomas, a ‘predominant pattern’ for subtyping invasive
adenocarcinoma could be reproduced with high concordance among
pathologists [31]. Recognition of the adenocarcinoma in-situ pattern
was more problematic [33]. On the contrary, a recent study showed
good agreement between observers when classifying tumors as AIS,
MIA and IA [32]. This study however was conducted in a large volume
practice with dedicated sub specialization and high level of expertise
in this subject [34]. It might be assumed that results would have been
different when applied to a more general pathology practice.
Possible consequences
The preoperative prediction of invasiveness is crucial in the
management of subsolid nodules, as these show different natural
histories: some nodules grow, some remain stable and grow after a
short period of time and others start to grow after many years (Figure
5). Until today, the behavior of these subsolid nodules remains
unclear. In a prospective study, the natural course of subsolid nodules
was evaluated. A total of 1229 subsolid nodules were included with a
mean prospective follow-up period of 4.3 years. Among the 1046 pure
GGNs, 1.2% developed into heterogeneous GGNs and 5.4% into partsolid
nodules. Among the 81 heterogeneous GGNs, 19.8% developed
into part-solid nodules. Invasive adenocarcinomas were diagnosed
only among the part-solid nodules, corresponding to 1% of all 1229
subsolid nodules [35]. Lung cancers associated with these subsolid
nodules have a high survival rate leading to concern about risk for
overdiagnosis. Adenocarcinomas manifesting as subsolid nodules
have longer volume doubling times compared to solid nodules.
Currently, there are no validated biomarkers or imaging features
available to predict growth or invasiveness. Lee “et al”. [36] found that
a history of prior lung cancer, a part-solid aspect and the diameter of
subsolid nodules were significant predictors for the growth of subsolid
nodules with solid parts smaller or equal than 5mm. Matsuguma “et
al”. [37] retrospectively correlated the growth of subsolid nodules
with the type of subsolid nodule, initial nodule size and history of
lung cancer. They found that a nodule size of more than 10mm and
a history of prior lung cancer were significant predictive factors of
growth in nonsolid nodules.
Some pure ground glass lesions might never become a part- solid nodule and some part-solid nodules might never evolve to an
invasive carcinoma. This low radiologic-pathologic correlation of
subsolid nodules hampers the search for biomarkers for growth and
invasiveness.
In addition to a risk of overdiagnosis, there is also a risk of
underdiagnosis. Part-solid nodules are prone to rigorous long-term
follow-up. When a solid component of more than 5mm is seen and
the lesion is evolving over time, surgery is recommended. In contrast,
pure ground glass lesions are not generally resected but followed until
they significantly grow or a solid component appears. As previously
mentioned, there are pure ground glass lesions that are not AAH or
AIS, but show an invasive component on pathological examination.
There currently are no biomarkers to predict invasiveness in pure
ground glass lesions. The clinical impact and impact on survival is
unclear and difficult to estimate, especially since these lesions have
an indolent nature.
Assessment of invasiveness is key to selection of management
and patient selection for surgery [38]. Preoperative classification of
the subtype is essential for patient management. Whereas lobectomy
remains the standard of care for stage I invasive adenocarcinoma,
lesser resections can be a valid alternative in patients presenting
with part-solid nodules. Numerous uncontrolled studies have shown
that for small adenocarcinomas, wedge excision or segmentectomy
is equivalent to lobectomy in terms of cancer-specific survival [39-
44]. Sparing lung parenchyma is vital in an elderly population with
impaired lung function. Additionally patients presenting with these
adenocarcinoma subtype lesions often have multiple ground glass
nodules. Since these lesions are likely to become invasive, sparing
lung parenchyma is important keeping in mind that future surgeries
might be necessary [45]. Overdiagnosis of invasive adenocarcinoma
that turns out to be MIA on pathology, will result in unnecessary
lobectomies instead of wedge excisions.
Imaging solutions are on the way
What remains a challenge to the human eye and prone to
variability might be solved by computer-aided technologies. In
radiology, research in the field of quantitative analysis is expanding
and certainly will be promising for the future. Correct classification
of a nodule into solid or subsolid (pure ground glass or part-solid)
is the first step in nodule management but is prone to variability.
Jacobs “et al”. [46] suggest a potential role for a Computer-Aided
Diagnosis (CAD) system in classifying pulmonary nodules. The
borders of nodules with a ground glass component are often hazy,
making correct measurement challenging and prone to considerable
variability. Decrease in dose will increase the noise, leading to an even
larger increase in variability or measurement error. Semi-automatic
segmentation of nodules and volumetric assessment of both ground
glass and solid component might be a first step in standardization
[47,48]. As mentioned, some pure ground glass nodules on imaging
show an invasive component on histopathology. For the moment,
visual assessment cannot differentiate pure GGNs with pathological
invasiveness from GGNs that are AAH or AIS on histopathology.
Eguchi “et al”. [49] found that the mean CT attenuation could be useful
in predicting invasive growth in pure GGN’s. Nodule characteristics
such as ground-glass opacity ratio and tumor disappearance rate
might correlate better with the IASLC/ATS/ERS classification than
the current radiologic-pathologic correlation. Quantitative analysis
of CT imaging metrics such as mass, skewness/kurtosis, attenuation,
texture parameters, might be able to differentiate invasive
adenocarcinoma from AIS or MIA among lesions that appear as
pure GGN with little solid component on CT [51,52]. Chae “et al”.
[53] showed that texture analysis could be promising to differentiate
invasive adenocarcinomas from pre-invasive lesions in subsolid
lesions. Iodine mapping may improve early recognition of invasive
adenocarcinoma appearing as pure GGN or part-solid nodules with
little solid component [54]. Assessing attenuation values might also
be able to predict change or rate of growth in subsolid nodules [55].
Measurement of mass in subsolid nodules can enable detection of
growth earlier than the human eye can detect discrete increase in
density. Moreover, mass measurement is less prone to variability
compared to visual assessment [56].
Computer-aided detection and classification, volumetric
measurement, texturizing and mass measurement might overcome
the problems of variability and are a good step towards a more
standardized approach of radiologic-pathologic correlation.
Conclusion
Abandoning the confusing term BAC and introducing the 2011 IASLC/ATS/ERS classification system was a milestone in the standardization of classification lung adenocarcinoma and its precursors. The radiologic-pathologic correlation of subsolid nodules holds imperfections, certainly creating room for improvement. Computer aided techniques and quantitative CT analysis is on the rise and definitely will have an impact on characterization and management of subsolid nodules. Lowering variability and increasing standardization, will make prospective studies regarding radiologicpathologic correlation in subsolid nodules more accurate and reliable. Computer-aided technologies might also give an insight in the natural course of progression, an area that upon today remains unresolved. Patient care will benefit from early recognition of invasiveness. More accurate radiologic-pathologic correlation will lower the risk of overdiagnosis, will aid in more optimal patient selection for surgical treatment as well as selection of the most beneficial and valid oncological surgical procedure. Quantification and standardization will be fundamental for answering the numerous remaining questions and addressing the uncertainties.
References
- Horeweg N, van der Aalst CM, Thunnissen E, Nackaerts K, Weenink C, Groen HJM, et al. Characteristics of Lung Cancers Detected by Computer Tomography Screening in the Randomized NELSON Trial. Am J Respir Crit Care Med. 2013; 187(8): 848–854.
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011; 365(5): 395–409.
- Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma. J Thorac Oncol. 2011; 6(2): 244–285.
- Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol. 2015; 10(9): 1243–60.
- Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008; 246(3): 697–722.
- Jones KD. Whence lepidic?: the history of a Canadian neologism. Arch Pathol Lab Med. 2013; 137: 1822-1824.
- Henschke CI, Yip R, Smith JP, Wolf AS, Flores RM, Liang M, et al. CT Screening for Lung Cancer: Part-Solid Nodules in Baseline and Annual Repeat Rounds. American J Roentgenol. 2016; 207(6): 1176-1184.
- Yip R, Yankelevitz DF, Hu M, Li K, Xu DM, Jirapatnakul A, et al. Lung Cancer Deaths in the National Lung Screening Trial Attributed to Nonsolid Nodules. Radiology. 2016; 281(2): 589-596.
- van Klaveren RJ, Oudkerk M, Prokop M, Scholten ET, Nackaerts K, Vernhout R, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med. 2009; 361(23): 2221–2229.
- Henschke CI, Yankelevitz DF, Mirtcheva R, McGuinness G, McCauley D, Miettinen OS, et al. CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. American Journal of Roentgenology. 2002; 178(5): 1053–1057.
- Russell PA, Wainer Z, Wright GM, Daniels M, Conron M, Williams RA. Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol. 2011; 6(9): 1496–1504.
- Saji H, Matsubayashi J, Akata S, Shimada Y, Kato Y, Kudo Y, et al. Correlation between whole tumor size and solid component size on high-resolution computed tomography in the prediction of the degree of pathologic malignancy and the prognostic outcome in primary lung adenocarcinoma. Acta Radiol. 2015; 56(10): 1187–1195.
- Warth A, Muley T, Meister M, Stenzinger A, Thomas M, Schirmacher P, et al. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol. 2012; 30(13): 1438–1446.
- Burt BM, Leung AN, Yanagawa M, Chen W, Groth SS, Hoang CD, et al. Diameter of Solid Tumor Component Alone Should be Used to Establish T Stage in Lung Adenocarcinoma. Ann Surg Oncol. 2015; 22 Suppl 3: S1318–S1323.
- Hwang EJ, Park CM, Ryu Y, Lee SM, Kim YT, Kim Y-W, et al. Pulmonary adenocarcinomas appearing as part-solid ground-glass nodules: is measuring solid component size a better prognostic indicator? Eur Radiol. 2015; 25(2): 558–567.
- Tsutani Y, Miyata Y, Nakayama H, Okumura S, Adachi S, Yoshimura M, et al. Prognostic significance of using solid versus whole tumor size on high-resolution computed tomography for predicting pathologic malignant grade of tumors in clinical stage IA lung adenocarcinoma: a multicenter study. J Thorac Cardiovasc Surg. 2012; 143(3): 607–612.
- Travis WD, Asamura H, Bankier AA, Beasley MB, Detterbeck F, Flieder DB, et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol. 2016; 11(8): 1204–1223.
- MacMahon H, Austin JHM, Gamsu G, Herold CJ, Jett JR, Naidich DP, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005; 237(2): 395–400.
- Naidich DP, Bankier AA, MacMahon H, Schaefer-Prokop CM, Pistolesi M, Goo JM, et al. Recommendations for the Management of Subsolid Pulmonary Nodules Detected at CT: A Statement from the Fleischner Society. Radiology. 2013; 266(1): 304–317.
- MacMahon H, Naidich DP, Goo JM, Lee KS, Leung ANC, Mayo JR, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology. 2017: 161659.
- Baldwin DR, Callister ME. The British Thoracic Society guidelines on the investigation and management of pulmonary nodules. Thorax. 2015; 70(8): 794–798.
- Detterbeck FC, Marom EM, Arenberg DA, Franklin WA, Nicholson AG, Travis WD, et al. The IASLC Lung Cancer Staging Project: Background Data and Proposals for the Application of TNM Staging Rules to Lung Cancer Presenting as Multiple Nodules with Ground Glass or Lepidic Features or a Pneumonic Type of Involvement in the Forthcoming Eighth Edition of the TNM Classification. J Thorac Oncol. 2016; 11(5): 666–680.
- American College of Radiology [Internet]. Lung CT Screening Reporting and Data System (Lung-RADS™) - American College of Radiology. [cited 2017Mar25].
- Honda T, Kondo T, Murakami S, Saito H, Oshita F, Ito H, et al. Radiographic and pathological analysis of small lung adenocarcinoma using the new IASLC classification. Clin Radiol. 2013; 68(1): e21–e26.
- Cohen JG, Reymond E, Lederlin M, Medici M, Lantuejoul S, Laurent F, et al. Differentiating pre- and minimally invasive from invasive adenocarcinoma using CT-features in persistent pulmonary part-solid nodules in Caucasian patients. Eur J Radiol. 2015; 84(4): 738–744.
- Fournel L, Etienne H, Mansuet Lupo A, Damotte D, Rouquette A, Revel M-P, et al. Correlation between radiological and pathological features of operated ground glass nodules. Eur J Cardiothorac Surg. 2017; 51(2): 248–254.
- Eguchi T, Yoshizawa A, Kawakami S, Kumeda H, Umesaki T, Agatsuma H, et al. Tumor size and computed tomography attenuation of pulmonary pure ground-glass nodules are useful for predicting pathological invasiveness. PLoS ONE. 2014; 9(5): e97867.
- Lee KH, Goo JM, Park SJ, Wi JY, Chung DH, Go H, et al. Correlation between the size of the solid component on thin-section CT and the invasive component on pathology in small lung adenocarcinomas manifesting as ground-glass nodules. J Thorac Oncol. 2014; 9(1): 74–82.
- van Riel SJ, Sánchez CI, Bankier AA, Naidich DP, Verschakelen J, Scholten ET, et al. Observer Variability for Classification of Pulmonary Nodules on Low-Dose CT Images and Its Effect on Nodule Management. Radiology. 2015 Dec;277(3):863–71.
- Singh S, Pinsky P, Fineberg NS, Gierada DS, Garg K, Sun Y, et al. Evaluation of reader variability in the interpretation of follow-up CT scans at lung cancer screening. Radiology. 2011; 259(1): 263–270.
- Penn A, Ma M, Chou BB, Tseng JR, Phan P. Inter-reader variability when applying the 2013 Fleischner guidelines for potential solitary subsolid lung nodules. Acta Radiol. 2015; 56(10): 1180–1186.
- Ridge CA, Yildirim A, Boiselle PM, Franquet T, Schaefer-Prokop CM, Tack D, et al. Differentiating between Subsolid and Solid Pulmonary Nodules at CT: Inter- and Intraobserver Agreement between Experienced Thoracic Radiologists. Radiology. 2016; 278(3): 888-8896.
- Thunnissen E, Beasley MB, Borczuk AC, Brambilla E, Chirieac LR, Dacic S, et al. Reproducibility of histopathological subtypes and invasion in pulmonary adenocarcinoma. Mod Pathol. 2012; 25(12): 1574–1583.
- Boland JM, Froemming AT, Wampfler JA, Maldonado F, Peikert T, Hyland C, et al. Adenocarcinoma in situ, minimally invasive adenocarcinoma, and invasive pulmonary adenocarcinoma--analysis of interobserver agreement, survival, radiographic characteristics, and gross pathology in 296 nodules. Hum Pathol. 2016; 51: 41–50.
- Kakinuma R, Noguchi M, Ashizawa K, Kuriyama K, Maeshima AM, Koizumi N, et al. Natural History of Pulmonary Subsolid Nodules: A Prospective Multicenter Study. J Thorac Oncol. 2016; 11(7): 1012–1028.
- Lee JH, Park CM, Lee SM, Kim H, McAdams HP, Goo JM. Persistent pulmonary subsolid nodules with solid portions of 5 mm or smaller: Their natural course and predictors of interval growth. Eur Radiol. 2016; 26(6): 1529–1537.
- Matsuguma H, Mori K, Nakahara R, Suzuki H, Kasai T, Kamiyama Y, et al. Characteristics of subsolid pulmonary nodules showing growth during follow-up with CT scanning. Chest. 2013; 143(2): 436–443.
- Pedersen JH, Rzyman W, Veronesi G, D'Amico TA, Van Schil P, Molins L, et al. Recommendations from the European Society of Thoracic Surgeons (ESTS) regarding computed tomography screening for lung cancer in Europe. Eur J Cardiothorac Surg. 2017; 51(3): 411–420.
- Kodama K, Higashiyama M, Okami J, Tokunaga T, Imamura F, Nakayama T, et al. Oncologic Outcomes of Segmentectomy Versus Lobectomy for Clinical T1a N0 M0 Non-Small Cell Lung Cancer. Ann Thorac Surg. 2016; 101(2) :504–511.
- McDonald F, De Waele M, Hendriks LEL, Faivre-Finn C, Dingemans A-MC, Van Schil PE. Management of stage I and II nonsmall cell lung cancer. Eur Respir J. 2017; 49(1): 1600764.
- Altorki NK, Kamel MK, Narula N, Ghaly G, Nasar A, Rahouma M, et al. Anatomical Segmentectomy and Wedge Resections Are Associated with Comparable Outcomes for Patients with Small cT1N0 Non-Small Cell Lung Cancer. J Thorac Oncol. 2016; 11(11): 1984–1992.
- Cox ML, Yang CJ, Speicher PJ, Anderson KL, Fitch ZW, Gu L, Davis RP et al. The role of extent of surgical resection and lymph node assessment for clinical stage I pulmonary lepidic adenocarcinoma: An analysis of 1,991 patients. J Thorac Oncol. 2017; 12(4): 689-696.
- Tsutani Y, Miyata Y, Nakayama H, Okumura S, Adachi S, Yoshimura M, et al. Oncologic outcomes of segmentectomy compared with lobectomy for clinical stage IA lung adenocarcinoma: propensity score-matched analysis in a multicenter study. J Thorac Cardiovasc Surg. 2013; 146(2): 358–364.
- Cao C, Gupta S, Chandrakumar D, Tian DH, Black D, Yan TD. Meta-analysis of intentional sublobar resections versus lobectomy for early stage non-small cell lung cancer. Ann Cardiothorac Surg. 2014; 3(2): 134–141.
- Rocco G, Allen MS, Altorki NK, Asamura H, Blum MG, Detterbeck FC, et al. Clinical statement on the role of the surgeon and surgical issues relating to computed tomography screening programs for lung cancer. Anna Thorac Surg. 2013; 96(1): 357–360.
- Jacobs C, van Rikxoort EM, Scholten ET, de Jong PA, Prokop M, Schaefer-Prokop C, et al. Solid, part-solid, or non-solid?: classification of pulmonary nodules in low-dose chest computed tomography by a computer-aided diagnosis system. Invest Radiol. 2015; 50(3): 168–173.
- Scholten ET, Jacobs C, van Ginneken B, Willemink MJ, Kuhnigk J-M, van Ooijen PMA, et al. Computer-aided segmentation and volumetry of artificial ground-glass nodules at chest CT. AJR Am J Roentgenol. 2013; 201(2): 295–300.
- Scholten ET, de Jong PA, Jacobs C, van Ginneken B, van Riel S, Willemink MJ, et al. Interscan variation of semi-automated volumetry of subsolid pulmonary nodules. Eur Radiol. 2015; 25(4): 1040–1047.
- Eguchi T, Kondo R, Kawakami S, Matsushita M, Yoshizawa A, Hara D, et al. Computed tomography attenuation predicts the growth of pure ground-glass nodules. Lung Cancer. 2014; 84(3): 242–247.
- Takahashi M, Shigematsu Y, Ohta M, Tokumasu H, Matsukura T, Hirai T. Tumor invasiveness as defined by the newly proposed IASLC/ATS/ERS classification has prognostic significance for pathologic stage IA lung adenocarcinoma and can be predicted by radiologic parameters. J Thorac Cardiovasc Surg. 2014; 147(1): 54–59.
- Son JY, Lee HY, Lee KS, Kim J-H, Han J, Jeong JY, et al. Quantitative CT analysis of pulmonary ground-glass opacity nodules for the distinction of invasive adenocarcinoma from pre-invasive or minimally invasive adenocarcinoma. PLoS ONE. 2014; 9(8): e104066.
- Li Q, Fan L, Cao E-T, Li Q-C, Gu Y-F, Liu SY. Quantitative CT analysis of pulmonary pure ground-glass nodule predicts histological invasiveness. Eur J Radiol. Elsevier; 2017; 89: 67–71.
- Chae H-D, Park CM, Park SJ, Lee SM, Kim KG, Goo JM. Computerized Texture Analysis of Persistent Part-Solid Ground-Glass Nodules: Differentiation of Preinvasive Lesions from Invasive Pulmonary Adenocarcinomas. Radiology. 2014; 273(1): 285–293.
- Son JY, Lee HY, Kim J-H, Han J, Jeong JY, Lee KS, et al. Quantitative CT analysis of pulmonary ground-glass opacity nodules for distinguishing invasive adenocarcinoma from non-invasive or minimally invasive adenocarcinoma: the added value of using iodine mapping. Eur Radiol. 2015; 26(1): 43–54.
- Bak SH, Lee HY, Kim J-H, Um S-W, Kwon OJ, Han J, et al. Quantitative CT Scanning Analysis of Pure Ground-Glass Opacity Nodules Predicts Further CT Scanning Change. Chest. 2016; 149(1): 180–191.
- de Hoop B, Gietema H, van de Vorst S, Murphy K, van Klaveren RJ, Prokop M. Pulmonary ground-glass nodules: increase in mass as an early indicator of growth. Radiology. 2010; 255(1): 199–206.