Editorial
99mTc-MDM SPECT Offers ‘Standard of Diagnostic Care’ in the Management of Glioma
Nisha Rani1, Puja Hazari2, Anil K Mishra2 and Baljinder Singh1*
11Department of Nuclear Medicine, PGIMER, India
2Division of Radiopharamcy and Cyclotron, INMAS, India
*Corresponding author: Baljinder Singh, Department of Nuclear Medicine & PET, PGIMER, Chandigarh-160012, India
Published: 31 Mar, 2017
Cite this article as: Rani N, Hazari P, Mishra AK, Singh B.
99mTc-MDM SPECT Offers ‘Standard of
Diagnostic Care’ in the Management of
Glioma. Clin Oncol. 2017; 2: 1252.
Editorial
Glioblastoma Multiforme (GBM) is the most common primary brain tumor in adults. GBM
is characterized by their appearance as solid tumours and mostly with the presence of a central
necrosis. Although, GBM carries very poor prognosis but the management of the patients with
the introduction of External Beam Radiation Therapy (EBRT) as adjunct to surgical resection and
concomitant adjuvant chemotherapy has improved their prognosis significantly [1].
GBM tumours often recur despite using a multi-disciplinary approach for their treatment.
These combinatory therapies undoubtedly deliver high amount of radiation dose that kill tumour
cells within a target region but is often associated with undesirable side effects. Delayed radiation
necrosis is one the most prominent side effects of this therapeutic modality that can manifest as a
progressive contrast enhancement on follow-up Magnetic Resonance (MR) images. This does not
allow a clear differentiation of necrosis from tumour recurrence which remains a clinical dilemma
for the treating oncologists [1,2]. Moreover, due to the heterogeneous nature, there is a great interest
in increasing the radiation dose to certain portion of the tumour while sparing normal tissue
adjacent to tumor area [3].
Traditionally, the gold standard method to distinguish between radiation necrosis and recurrent
tumour is a repeat (post-surgical) histological confirmation by biopsy or surgical resection which
often is difficult especially in deep seated brain tumours. So, there is a need for a non-invasive imaging
technique for accurate differentiation of tumour necrosis from recurrence as the conventional CT
and MRI techniques may have conflicting results [4]. Advance MR techniques such as perfusion
and diffusion MR Spectroscopy (MRS), Dynamic Susceptibility Contrast-Enhanced (DSCE) and
SPECT/ PET imaging have been shown to be clinically useful in the detection of recurrent/residual
tumour [5,6,7]. As malignant cells display an increased rate of amino acid uptake and metabolism,
thus Positron Emission Tomography (PET) using radiolabeled amino acids holds great promise
in detection of glioma recurrence/residual/necrosis. In particular, 11C-L-methionine PET (MET
PET), O-(2-18F-fluoroethyl)-L-tyrosine (18F-FET) PET provides useful information about glioma metabolism and has emerged as a promising tool in the management
of glioma. Therefore, the amino acid based PET imaging combined
with conventional imaging modalities can provide complementary
diagnostic and therapeutic information and also can guide selective
target tissue sampling or resection [6,8].
The widely used MET PET is limited by short half-life of 20-min
of 11C and requires in-house cyclotron facility. Furthermore, the
radiolabeling of PET based tracers for glioma imaging is cumbersome
and causes high radiation burden to the patients. Therefore, there is a
clinical need for developing a more practical imaging method which
can offer a cost effective substitute to glioma PET imaging. We have
developed 99mTc-DTPA-bis methionine (99mTc-MDM) as single vial
lyophilized kits ready to label ‘with 99mTc’ and have standardized
the clinical use of this imaging technique in glioma SPECT imaging
[9,10]. Tumor uptake of 99mTc-MDM is similar (transport through
LAT-1) to that of 11C-MET. 99mTc-MDM concentrating preferentially
in the metabolically active post surgical, post chemo-radiotherapy
residual GBM disease and in the primary GBM tumor is presented
in Figures 1 and 2.
Detection of metabolically active primary or residual/recurrent
tumor and accurate delineation of tumor boundaries has clinical
applications for targeted biopsies or radiation treatment planning.
99mTc-MDM SPECT may serve as a prognostic marker for glioma
management including staging, restaging, defining Biological Tumor
Volume (BTV) for high-dose radiotherapy boost and post treatment
assessment and may provide a cost effective substitute for amino acid
based PET imaging.
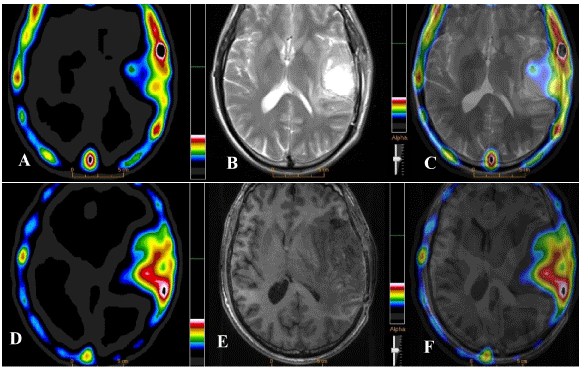
Figure 1
Figure 1
An increased uptake of 99mTc-MDM on SPECT image (A) in a 48 years old male GBM patient, contrast
enhancement on CE-MR and fused SPECT/MR images (B & C) shown post surgical/chemo-radiotherapy (at
3-months) residual disease in the left temporal lobe. The subsequent SPECT (D), DSCE MR (E) and fused
SPECT/MR images at 6-months follow-up indicated a significant disease progression in the corresponding
region of the brain.
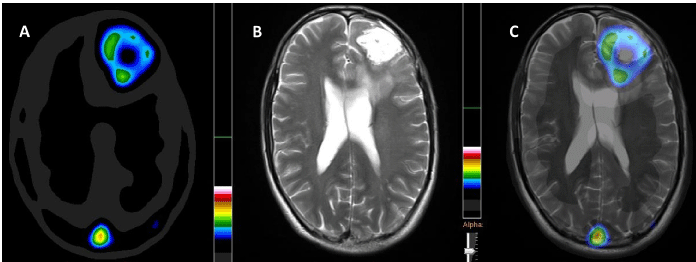
Figure 2
Figure 2
An increased 99mTc-MDM uptake SPECT (A), contrast enhancement
on CE-MR and fused SPECT/MR images (B & C) indicating metabolically
active GBM disease pre-operatively in a 39 years old male. 99mTc-methionine
uptake (A) demonstrates metabolically active tumor in the rim with central
necrotic mass. CE-MRI (B) indicates homogenous contrast enhancement in
the entire tumor bed.
References
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-96.
- Shah AH, Snelling B, Bregy A, Patel PR, Tememe D, Bhatia R et al. "Discriminating radiation necrosis from tumor progression in gliomas: a systematic review what is the best imaging modality?" J. Neurooncol. 2013;112(2):141-152.
- Iuchi T, Hatano K, Narita Y, Kodama T, Yamaki T, Osato K. Hypofractionated high-dose irradiation for the treatment of malignant astrocytomas using simultaneous integrated boost technique by IMRT. Int J Radiat Oncol Biol Phys. 2006;64(5):1317-1324.
- Deng SM, Zhang B, Wu YW, Zhang W, Chen YY. Detection of glioma recurrence by 11C-methionine positron emission tomography and dynamic susceptibility contrast-enhanced magnetic resonance imaging: a meta-analysis. Nucl Med Commun. 2013;34(8):758-66.
- Jenkinson MD, Du Plessis DG, Walker C, Smith TS. Advanced MRI in the management of adult gliomas. Br J Neurosurg. 2007;21(6):550-61.
- Chen W. Clinical applications of PET in brain tumors. J Nucl Med. 2007;48(9):1468-81.
- Alexiou GA, Zikou A , Tsiouris S, Goussia A, Kosta P, Papadopoulos A et al. Comparison of diffusion tensor, dynamic susceptibility contrast MRI and 99mTc-Tetrofosmin brain SPECT for the detection of recurrent highgrade glioma. J Magn Reson Imaging. 2014;32:854–859.
- Weber WA, Wester HJ, Grosu AL, Herz M, Dzewas B, Feldmann HJ,et al. O-(2-[18 F] fluoroethyl)-L-tyrosine and L-[methyl-11 C] methionine uptake in brain tumours: initial results of a comparative study. Eur J Nucl Med Mol Imaging. 2000;27(5):542-549.
- Hazari PP, Shukla G, Goel V, Chuttani K, Kumar N, Sharma R, et al. Synthesis of specific SPECT-radiopharmaceutical for tumor imaging based on methionine: 99mTc-DTPA-bis (methionine). 'Bioconjugate Chem. 2010;21(2):229-39.
- Singh B, Kumar N, Sharma S, Watts A, Hazari PP, Rani N, Vyas S, et al. 99mTc-MDM Brain SPECT for the Detection of Recurrent/Remnant Glioma—Comparison With ceMRI and 18F-FLT PET Imaging: Initial Results. Clin Nucl Med. 2015;40(10):e475-e479.