Research Article
Response-Based Intensive Induction Chemotherapy of Curative Approach in Elderly Acute Myelogenous Leukemia Patients in Single Institution
Yoshiko Saito1,2*, Yoshiro Uzuka1,Yhuka Takahashi1 and Mari Ohtsuka1
Department of Food and Nutrition, Miyagi Women’s University, Japan
*Corresponding author: Yoshiko Saito, Department of Food and Nutrition, Miyagi Women’s University, Sendai Blood Disease Center, 28-3 Tomita aza Minamino-nishi, Taihakuku, Sendai City, Miyagi, Japan
Published: 20 Mar, 2017
Cite this article as: Saito Y, Uzuka Y,Takahashi Y, Ohtsuka
M. Response-Based Intensive Induction
Chemotherapy of Curative Approach in
Elderly Acute Myelogenous Leukemia
Patients in Single Institution. Clin Oncol.
2017; 2: 1228.
Abstract
To date, about 60-80% of adults with previously untreated de novo Acute Myeloid Leukemia (AML)
enter Complete Remission (CR) when treated with standard regimen. However, such responses are
rarely durable and relapsed when conventional therapy is administered. We aimed to establish a
curable treatment protocol because prognosis after relapse is poor and fatal.
Over the last decades, progress in treatment for patients with AML, based on risk-directed
stratification strategy, had brought large benefit to many patients. On the other hand, our treatment
strategy was based on the fact that the assessment of treatment effect might help to define the
prognosis of patient and possibly.
A total of 88 patients with de novo untreated AML treated between March 1995 and February
2011 was analyzed. Response-based intensive induction chemotherapy: single induction treatment
consisted of priming standard regimen and additional induction regimen, which was continuedtill
complete clearance of marrow leukemic blasts.
Results: By single induction strategy, CR was obtained in 21/21(100 %) of younger patients and in
19/20(95%) of elderly.8-week mortality was 0/21(0%) and1/20(5%) respectively. By an additional
chemotherapy course, 8-week mortality was 7/24 (29.2%) in elderly. Differences between the single
induction course and an additional courses at univariate analysis were statistically significant for CR
(p = 0.0053) and for 8 week mortality (p = 0.0031).
Multivariate analysis of prognostic factors identified consistent independent poor prognostic
factor for CR, 8-week mortality, and survival. These included age, unfavorable karyotypes, poor
performance status, and abnormal organ functions. It was suggested that all risk factors may be
overcome by the single induction strategy for patients with AML.
There was no co-relationship to the Charlson Comorbidity Index and the 8-week mortality. WT-1
measurements produced a dilemma, AML1/MTG8 and CBFβ/MYH11gene mutation measurement
was the result which could be reflected in the chemotherapy.
Conclusions: Our unique “response-based single induction strategy” produces high CR rates over
95% and long-term Disease-Free Survival (DFS) over 70%. The intensified treatment was well
tolerated in elderly as well as younger patients.
Keywords: Acute myeloid leukemia; Intensive induction chemotherapy; Blast cell clearance; End point of treatment; Elderly, MRD
Introduction
Many novel molecular targeting agents have been developed for acute myeloid leukemia but,
still now, frontline therapy of acute myeloid leukemia has remained largely unchanged excluding
the acute promyelocytic leukemia (APL) for several decades [1]. Standard regimen of Acute Myeloid
Leukemia (AML) using anthracyclines and cytarabine for induction (3+7 regimen followed by
various post-remission therapy has remained minor changed produce CR rates of 60% to 80%,
With less than 20% of all patients achieving long-tsserm Disease-Free Survival (DFS) [2-4]. Over
the last decade intensive induction chemotherapy produced the CR in the majority of adult patients
with AML [5,6]. Despite these improvements, the long-term survival rate among patients who are
less than 60 years of age is only 40%, and less than 10% of elderly patients with AML [2,7-10].
Unfortunately, the increase of dose intensity in elderly patients [3,5,11] is characterized by an increase of CR rate and substantial increase of induction mortality rate [3,5-7].
There are several host and disease related predictive factors, among
which adverse cytogenetics [6,12-15] and the presence of transmembrane
transport proteins [16]. Recent prospective randomized
studies clearly demonstrate that elderly patients benefit from more
intensive induction therapy [7,13,17-20] and particularly from fulldose
application of anthracycline and possibly also cytarabine [6].
Kantarjean et al. [21] proposed prognostic models for CR,
induction mortality and survival rates in elderly AML to establish
baseline chemotherapy. They emphasized that cytogenetic studies
have acquired major prognostic importance in the therapy of AML.
The recent report of the Cancer and Leukemia Group B trials (9222)
[22] tested the treatment intensification of AML in first remission with
multiple sequential chemotherapy and high-dose cytarabine alone in
younger patients, but there was no difference in DFS between the 2
regimens. Recent progress in treatment for patients with AML, based
on risk-directed stratification strategy had brought large benefit to
many patients [3,6,21]. Finally, recent report showed that intensity of
the chemotherapy might be adapted to individual patient [23]. Some
data showed no benefit of high-dose therapy and an autotransplant
[24], while there is also the report that the high risk nature of the
patients were favorable [1].
Our aim is to report remarkable results on intensive single
induction strategy which provides insights to chemotherapeutic
modality predictive for CR rates, induction mortality and survival.
Intensified induction therapy could overcome almost all these adverse
factors [25].
Minimal Residual Disease (MRD) analysis based on quantitative
PCR of common fusion or mutated genes is gaining acceptance as
a risk stratification tool and as a measure of impending relapse
in AML. MRD is of clinical value in the assessment of response
to chemotherapy, predicting relapse, and guiding therapeutic
intervention [26-30]. Along with this report the importance of
intensive remission induction therapy, for the determination of post
remission and maintenance chemotherapy duration, the WT-1 and
the fusion gene were measured with treatment courses [27,29,30].
Material and Methods
Patients
Eighty-eight consecutive patients age 16-88 years with de novo
previously untreated AML were treated on intensive chemotherapy
in Sendai Blood Disorder Center (SBDC) during March 1995 and
February 2011. Patients with controlled co-morbid conditions were
not excluded in this study. Patients with APL were excluded from this
study. All patients provided written informed consent. Morphologic
diagnosis of AML was made on May-Giemsa stained blood and bone
marrow smears, and the diagnosis was confirmed by appropriate
cytochemical staining, immunophenotyping by multicolored
flow cytometry, and cytogenetics of leukemic cells. The disease
was classified according to the French-American- British (FAB)
classification system [31-33]. Performance status was assessed with
the WHO criteria [34].
Karyotype was classified according to the International System for
Cytogenetic Nomenclature. Favorable karyotypes were those with the
abnormalities t(8;21),t(15;17) and inv (16). Unfavorable karyotypes
were those with monosomy of chromosomes 5 or 7, deletion of
the long arm of chromosome 5, abnormalities of the long arm of
chromosome 3 or a complex karyotype (definedas more than three abnormalities). Patients with normal karyotype or with abnormalities
other than those defined as favorable or unfavorable were classified as
the intermediate cytogenetic group.
Treatment
At first, all consecutive patients received the priming induction
therapy consisting of 40mg/m2 daunorubicin by intravenous infusion
on days 1-3 and 120 mg/m2/day cytarabine by intravenous infusion
daily by every 12 hour infusion on days 1-7. On days 8 of induction
therapy, the bone marrow was examined for the nucleated cell counts.
When the result was the presence of blast cells, in response-based
intensive induction strategy (SI), the addition of the medicine was
carried out. That was continued till complete clearance of marrow
leukemic blasts was obtained. Induction treatment was completed
when the bone marrow nucleated cells <0.8 X 109 /L (corrected
count) [35] in marrow aspirate with spicules, clearance of almost all
blast cells (<2%) and the peripheral blood WBC count <0.6 X 109/L
(end point for the completion of induction therapy-target point)
was obtained. If the induction therapy could not reach to the target
point, the induction course was subsequently continued till days 11
with daunorubicin 40mg/m2/day and cytarabine 120mg/m2/day as to
reach to the target point by monitoring with every other day bone
marrow examination. Generally, induction course was discontinued
at 12 days to avoid later severe side-effects, and a second course of
induction was usually not needed. After reaching to the target point,
generally the bone marrow blast regeneration (>5% blast) did not
reappear in weekly bone marrow sampling till CR. All younger
patients who went into CR received 5 courses of consolidation
therapy consisting of the same regimens at equivalent dose for 7 days.
However in elderly patients 2-5 courses of consolidation therapy were
given adjusting to myelotoxicity. Maintenance treatment consisting
of 6 weekly courses of 30mg/m2/d daunorubicin on days1 and 5 and
70mg/m2/d cytarabine on days 1 to 5 was continued until molecular
CR (2-5 courses) or a relapse. Close supportive care was given. All
patients received intensive induction therapy in a laminar air flow
room. For patients with decreasing absolute neutrophil counts(ANC)
(<0.5 X 109/L), granulocyte colony stimulating factor (lenograstim:
5μg/kg/d or filgrastim: 6μg/kg/d ) was administered till recovery
to 1.0 X 109 /L ANC. Platelet transfusion was given for patients
with decreased platelet count <10.0-20.0 X 109/L with hemorrhagic
tendency. Supportive care included antibiotics and antifungal
prophylaxis, blood product support.
Response criteria
A complete remission requires normalization of bone marrow
with 5% or less blasts in aspirate samples with spicules and with a
count of 500 nucleated cells, and peripheral neutrophil counts 1 X
109/L or above, and platelet counts100x109/L or above. There should
be no blasts with Auer rods or persistence of extramedullary disease.
Partial remission was defined as the persistence of marrow blast
5-20%. Patients with <5% blasts but with a hypo-cellular marrow
precluding CR were also classified as being in PR. Failure was defined
as marrow blasts >20. Cytogenetic complete remission was defined as
reversion to normal karyotype at CR. Molecular CR was assessed using
automated quantitative Reverse Transcriptase Polymerase Chain
Reaction (RT-PCR) technique [36] in case with a specific gene maker.
Other molecular target, such as WT-1, was also assessed. Overall
survival was defined as the time from the start of induction therapy to
either death or last follow-up, censoring patients alive. Disease-Free
Survival (DFS) was defined as the time from CR to either relapse or death in first CR, or last follow-up, censoring patients alive in first
CR. 8-week mortality was related to treatment and/or hypoplasia.
Cardiotoxicity was assessed using conventional cardio-echography
and our original method: the phased tracking method [37,38].
Analysis for WT-1, AML1/MTG8 and CBFβ/MYH11 Gene
Mutations
Available bone marrow samples and peripheral blood samples
were obtained at diagnosis, post induction, post consolidation and
during treatment-free remission. Total RNA extracted from bone
marrow samples and peripheral blood samples by AGPC method.
WT-1, AML1/MTG8 and CBFβ/MYH11 were determined by RTPCR
(EYELA) and real-time PCR (an ABI PRISM7700).
Statistical analysis
Differences among categorical covariates were evaluated using
the chi-squared test. Response rates were compared in univariate
analysis by the chi-squared test. Survival and remission duration
curves were plotted by the Kaplan-Meier method and compared by
the log rank test.
Multivariate analysis [33] of prognostic factors used the logistic
regression methods for CR, induction mortality (8-week mortality)
and Cox proportional hazard method for survival with standard
methods using SAS ver. 8.02. Statistical significance is represented by
two sided p values.
Table 1
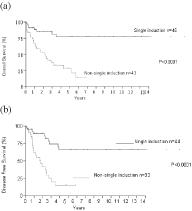
Figure 1
Figure 2
Figure 2
Non-single induction therapy compared with age category (a)
overall survival (b) disease free survival.
Results
Presentation features of the entire patients were shown in (Table
1). Median follow-up of surviving patients is 5.1 years (range 0.3-
14 years). Total of 88 patients were analyzed. Their median age was
66 years (range 16-88). Differences between the two groups for age,
karyotypes, and WBC except PS were not statistically different.
Response to therapy
CR rate was16/19 (84.2%) in younger and 17/24 (70.8%) in elderly
patients treated with non-single protocol (p = 0.0185) and 24/24 (100%) and 20/21 (95.2%) respectively in single induction group (p
= 0.2131). 8-week mortality was 0/19 and 7/24 (29.2%) respectively
in non-single induction group(p = 0. 0053) and 0/24 and 1/21 (4.8%)
respectively in single induction group (p = 0.213). Differences
between the two groups at univariate analysis were statistically
significant for CR (p = 0.0053) and for 8 week mortality (p = 0.0031).
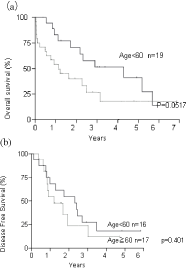
In non-single group, the overall survival was 41.0% in younger versus
13.3% in elderly (Figure 1a), and the 5-year DFS was 34.1% in younger
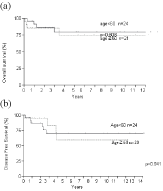
versus 11.9% in elderly (Figure 1b). In the single induction group the
5-year survival rate was 72.4 % in younger 76.2% in elderly (Figure
2a) and the 5-year DFS was 72.8% and 69.6 % respectively (Figure
2b). Differences between the two groups at univariate analysis there
were statistically significant differences for OS (p <0.0001) (Figure
3a) and DFS (p <0.0001) (Figure 3b). In non-single group, variables
influencing response were age, PS and karyotype. There were
significant differences in CR rates and substantial differences in longterm
survival between both groups. In the single induction group,
neither adverse cytogenetics nor PS were statistically significantly
different for predictive of CR rate and long-term survival (Table 2).
More than 60 years old 8-week mortality patients at eight, Wheatley
[39] index were all patients poor. Two patients in 8-weekmortality
with the Charlson comorbid index [40] were 3, other 6 were 0.
Toxicity
Toxicity showed in (Table 3). Hematologic toxicity was acceptable
with around 15 days to recover to >0.5 X 109/L ANC in both younger
adults and elderly patients among both groups(p = 0.2756). Median
time to achieve an unsupported platelet count >30 X 109/L was around
15 days except in elderly patients treated with single induction therapy
(p = 0.355). Severe Infectious complications (WHO grade 3,4) during
induction therapy, in non-single induction group were observed in
14/43 (32.6%), whereas only 2/45 (4.4%) in single induction group (p
= 0.0062). Cardiac functions were gradually decreased by the phased
tracking method examination [37]. 6 out of 28 relapsed patients were
fatal cardiotoxicity, but no relapsed patients were recovered cardiac
function without additional chemotherapy [38].
MRD levels in chemotherapy courses
WT-1 and hybrid genes levels in the bone marrow and the peripheral blood samples collected over the course of chemotherapy
were determined. WT-1 was expressed in 33 patients out of 39
at the time of diagnosis, 24 at the CR time and on 61.3% patients
was expressed during disease free. Hybrid genes were expressed in
all examined patients at the time of diagnosis. Patients sustained
complete remission were negative, becomes positive before relapse
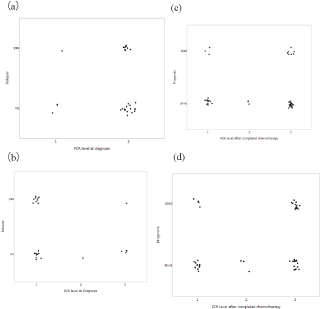
(Table 4). TheWT-1 levels decreased as the treatment progressed
were not predictive of the therapeutic efficacy, but re-elevation of the
hybrid-gene level suggested relapse (Figure 4a and b).
Figure 3
Figure 3
Single induction therapy compared with age category (A) overall
survival (B) disease free survival.
Table 2
Table 3
Table 4
Figure 4
Figure 4
Relationship between PCR level and outcomes.
(A) Relationship between WT-1 PCR level at diagnosis andrelapse
(B) PCR level at diagnosis of Hybrid genes vs. relapse
(C) WT-1 level after completed chemotherapy vs prognostic outcomes
(D) PCR levels of after completed chemotherapy vs prognostic outcomes
PCR levels 1: <10-5, 2: 10-5~10-3, 3: >10-3
Discussion
In1976, we proposed the DCMP 2-step Therapy [41] for induction
therapy of AML. In our early DCMP 2-step therapy, induction
treatment was divided into 2 courses with short interval (within
7-14 days). The CR rate of more than 80% obtained by this strategy
was one of excellent results at that decade [34]. Japanese Leukemia
Study Group (JALSG) has started on the conception of this
strategy with some modifications. However, long-term result was
disappointing (5-year DFS only 15%) by this strategy. The cause of
failure to achieve long-term outcome was considered as following:
in patients with AML, leukemic blast cell regeneration occurred
rapidly with changing cell-kinetics of leukemic blasts during and after
chemotherapy [42]. A malignant cell population that has survived
initial induction treatment might show resistance to chemotherapy
due to special genetic or kinetic changes. In contrast, early induction
therapy deals with naïve tumor cells possibly different from the
counterparts after chemotherapy in terms of their kinetic status and
sensitivity to chemotherapy [43]. In particular, older patients have
many problems due to leukemic cell drug resistance and decreased
tolerance of the side-effects of therapy. In 1991 we stared intensive
chemotherapy for elderly patients as well as younger adults with
AML using the response-oriented intensive induction chemotherapy
[44]. Remarkable improvement has been obtained in terms of CR,
however, improvement of long-term survival was not satisfactory.
Thus, we have started non-single induction therapy based on new
target point of induction therapy in 1995. However, even by this
method, high incidence of early chemotherapy death was not
avoided, particularly, in older patients. By this strategy intensive
induction was based on prolongation of duration and increasing of
dose of cytarabine alone. Based on many previous experiences and
according to some literaturs [23,45-47], we get the courage with the confidence that early blast cell clearance by early single induction
therapy is most important for both achievement of CR and longterm
outcome in both younger and elderly with AML. Thus we have
developed the unique responce0oriented intensive “single induction
strategy” with a curative intent for elderly as well as younger patients
with AML in 2000. The aim of the single induction strategy is to
minimize leukemic blasts by early blast clearance after one course of
induction therapy, suppression of early re-proliferation of residual
leukemic blasts during and after bone marrow aplasia, the decrease in
severe myelotoxicity induced by prolonged induction chemotherapy.
To suppress the re-proliferation of residual leukemic blast during
early induction therapy is essential for killing the naive tumor cells,
resulting in long-term remission or cure in patients with AML. It was
reported that elderly patients showed a great benefit from full-dose
application of standard induction regimen than from less intensive
chemotherapy [6,17,47]. In addition, toxicity may be reduced if
patients require no more than one cycle of induction therapy to
achieve complete remission [46,48]. Generally, hematopoietic
precursors are considered to be designed to survive the repeated
exposure to environmental toxins encountered during lifetime. As
demonstrated by marrow purging experiments, the hematopoietic
stem cell can survive an exposure to very large doses of cytotoxic
agents in vitro [21]. Thus hematopoietic precursors in the bone
marrow of elderly patients might survive early exposure to intensive
induction therapy. However, in the elderly patients, reserve function
of bone marrow might be reduced probably by the detrimental effects
of stem cell aging [45,49,50] and exhaustion of primitive stem cell
after chemotherapy. So, bone marrow stem cell function is more
vulnerable to delayed and repeated exposure to chemotherapeutic
regimens. In addition the leukemic cell in older patients arises from a
more proximal pluripotent stem cell in the hematopoietic hierarchy
than is the case for younger adults with the disease [3,5-7]. By its very nature, this proximal stem cell is intrinsically more resistant to
chemotherapy. We considered that chemo-therapeutic effects might
help to define the prognosis of the individual patient and possibly
might adapt the intensity of the chemotherapy to the individual
therapeutic response. The single induction strategy in very early
phase of treatment may not only minimize residual disease, but also
reduce substantial myelotoxicity. For this purpose, we decided the
end point of the first induction chemotherapy as to reach target point
by monitoring with repeated bone marrow examination at day 8-11
of induction treatment. Inclusively, our therapeutic modality is not
only adapted to individual response but also adapted to individual
toxicity for dose decision making which could overcome all adverse
factors. Elderly as well as younger adults have excellent outcomes. In
addition, single induction therapy introduces a more standardized
approach that achieves homogeneity in the quantity of induction
treatment for tumor cell burden. There was no patient’s refusal
of intensive chemotherapy. Our study shows that the aggressive
approach was feasible in all elderly as well as younger adults.For
patients wishing chemotherapy without feeling frailty until just
before leukemia diagnosis with a high possibility of early mortality
index and CCI [40], further research and investigation is necessary to
select chemotherapy.
The prognostic impact of the normalized bone marrow and
peripheral blood WT-1 levels at diagnosis, post-induction and post
intensification was limited on the our data but hybrid gene mutations
levels reflected the leukemic mass. But further investigation is needed
in order to reflect chemotherapy courses reduction or extension
[26,27,51]. MRD may serve as individualized chemotherapy options
of the consolidation and the maintenance therapy.
Conclusion
The most important thing to get a good long-term survival is to achieve a complete remission with one course of remission induction therapy. Our response-based intensive induction strategy is feasible and appropriate.
Acknowledgement
Give thanks to all the staff and patients who cooperated in this research.
References
- Seiter K, Ahmed N, Shaikh A, Baskind P, Liu D. CLAG-based induction therapy in previously untreated high risk acute myeloid leukemia patients. Leuk Res. 2016; 46: 74-78.
- Juliusson G, Antunovic P, Derolf A, Lehmann S, Möllgård L, Stockelberg D, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009; 113: 4179-4187.
- Kimby E, Nygren P, Glimelius B. A systematic overview of chemotherapy effects in acute myeloid leukaemia. Acta Oncol. 2001; 40: 231-252.
- Mayer RJ, Davis RB, Schiffer CA, Berg DT, Powell BL, Schulman P, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. Cancer and Leukemia Group B. N Engl J Med. 1994; 331: 896-903.
- Baudard M, Beauchamp-Nicoud A, Delmer A, Rio B, Blanc C, Zittoun R, et al. Has the prognosis of adult patients with acute myeloid leukemia improved over years? A single institution experience of 784 consecutive patients over a 16-year period. Leukemia. 1999; 13: 1481-1490.
- Rees JK, Gray RG, Wheatley K. Dose intensification in acute myeloid leukaemia: greater effectiveness at lower cost. Principal report of the Medical Research Council's AML9 study. MRC Leukaemia in Adults Working Party. Br J Haematol. 1996; 94: 89-98.
- Bennett JM, Young ML, Andersen JW, Cassileth PA, Tallman MS, Paietta E, et al. Long-term survival in acute myeloid leukemia: the Eastern Cooperative Oncology Group experience. Cancer. 1997; 80: 2205-2209.
- Derolf AR, Kristinsson SY, Andersson TM, Landgren O, Dickman PW, Bjorkholm M. Improved patient survival for acute myeloid leukemia: a population-based study of 9729 patients diagnosed in Sweden between 1973 and 2005. Blood. 2009; 113: 3666-3672.
- Schiffer CA, Dodge R, Larson RA. Long-term follow-up of Cancer and Leukemia Group B studies in acute myeloid leukemia. Cancer. 1997; 80: 2210-2214.
- Thein MS, Ershler WB, Jemal A, Yates JW, Baer MR. Outcome of older patients with acute myeloid leukemia: an analysis of SEER data over 3 decades. Cancer. 2013; 119: 2720-2727.
- Jabbour EJ, Estey E, Kantarjian HM. Adult acute myeloid leukemia. Mayo Clin Proc. 2006; 81: 247-260.
- Bloomfield CD, Lawrence D, Byrd JC, Carroll A, Pettenati MJ, Tantravahi R, et al. Frequency of prolonged remission duration after high-dose cytarabine intensification in acute myeloid leukemia varies by cytogenetic subtype. Cancer Res. 1998; 58: 4173-4179.
- Dastugue N, Payen C, Lafage-Pochitaloff M, Bernard P, Leroux D, Huguet-Rigal F, et al. Prognostic significance of karyotype in de novo adult acute myeloid leukemia. Leukemia. 1995; 9: 1491-1498.
- Grimwade D, Walker H, Harrison G, Oliver F, Chatters S, Harrison CJ, et al. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood. 2001; 98: 1312-1320.
- Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children's Leukaemia Working Parties. Blood. 1998; 92: 2322-2333.
- Menzin J, Lang K, Earle CC, Kerney D, Mallick R. The outcomes and costs of acute myeloid leukemia among the elderly. Arch Intern Med. 2002; 162: 1597-1603.
- Bishop JF, Matthews JP, Young GA, Bradstock K, Lowenthal RM. Intensified induction chemotherapy with high dose cytarabine and etoposide for acute myeloid leukemia: a review and updated results of the Australian Leukemia Study Group. Leuk Lymphoma. 1998; 28: 315-327.
- Büchner T, Berdel WE, Schoch C, Haferlach T, Serve HL, Kienast J, et al. Double induction containing either two courses or one course of high-dose cytarabine plus mitoxantrone and postremission therapy by either autologous stem-cell transplantation or by prolonged maintenance for acute myeloid leukemia. J Clin Oncol. 2006; 24: 2480-2489.
- Leith CP, Kopecky KJ, Godwin J, McConnell T, Slovak ML, Chen IM, et al. Acute myeloid leukemia in the elderly: assessment of multidrug resistance (MDR1) and cytogenetics distinguishes biologic subgroups with remarkably distinct responses to standard chemotherapy. A Southwest Oncology Group study. Blood. 1997; 89: 3323-3329.
- Weick JK, Kopecky KJ, Appelbaum FR, Head DR, Kingsbury LL, Balcerzak SP, et al. A randomized investigation of high-dose versus standard-dose cytosine arabinoside with daunorubicin in patients with previously untreated acute myeloid leukemia: a Southwest Oncology Group study. Blood. 1996; 88: 2841-2851.
- Kantarjian H, O'brien S, Cortes J, Giles F, Faderl S, Jabbour E, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer. 2006; 106: 1090-1098.
- Moore JO, George SL, Dodge RK, Amrein PC, Powell BL, Kolitz JE, et al. Sequential multiagent chemotherapy is not superior to high-dose cytarabine alone as postremission intensification therapy for acute myeloid leukemia in adults under 60 years of age: Cancer and Leukemia Group B Study 9222. Blood. 2005; 105: 3420-3427.
- Kern W, Haferlach T, Schoch C, Loffler H, Gassmann W, Heinecke A, et al. Early blast clearance by remission induction therapy is a major independent prognostic factor for both achievement of complete remission and long-term outcome in acute myeloid leukemia: data from the German AML Cooperative Group (AMLCG) 1992 Trial. Blood. 2003; 101: 64-70.
- Krug U, Berdel WE, Gale RP, Haferlach C, Schnittger S, Müller-Tidow C, et al. Increasing intensity of therapies assigned at diagnosis does not improve survival of adults with acute myeloid leukemia. Leukemia. 2016; 30: 1230-1236.
- von dem Borne PA, de Wreede LC, Halkes CJ, Marijt WA, Falkenburg JH, Veelken H. Effectivity of a strategy in elderly AML patients to reach allogeneic stem cell transplantation using intensive chemotherapy: Long-term survival is dependent on complete remission after first induction therapy. Leuk Res. 2016; 46: 45-50.
- Krauth MT, Alpermann T, Bacher U, Eder C, Dicker F, Ulke M, et al. WT1 mutations are secondary events in AML, show varying frequencies and impact on prognosis between genetic subgroups. Leukemia. 2015; 29: 660-667.
- Nomdedéu JF, Hoyos M, Carricondo M, Bussaglia E, Estivill C, Esteve J, et al. Bone marrow WT1 levels at diagnosis, post-induction and post-intensification in adult de novo AML. Leukemia. 2013; 27: 2157-2164.
- Shimada A, Taki T, Koga D, Tabuchi K, Tawa A, Hanada R, et al. High WT1 mRNA expression after induction chemotherapy and FLT3-ITD have prognostic impact in pediatric acute myeloid leukemia: a study of the Japanese Childhood AML Cooperative Study Group. Int J Hematol. 2012; 96: 469-476.
- Marani C, Clavio M, Grasso R, Colombo N, Guolo F, Kunkl A, et al. Integrating post induction WT1 quantification and flow-cytometry results improves minimal residual disease stratification in acute myeloid leukemia. Leuk Res. 2013; 37: 1606-1611.
- Shibasaki Y, Seki Y, Tanaka T, Miyakoshi S, Fuse K, Kozakai T, et al. The association of level of reduction of Wilms' tumor gene 1 mRNA transcript in bone marrow and outcome in acute myeloid leukemia patients. Leuk Res. 2015; 39: 667-671.
- Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol. 1976; 33: 451-458.
- Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposed revised criteria for the classification of acute myeloid leukemia. A report of the French-American-British Cooperative Group. Ann Intern Med. 1985; 103: 620-625.
- Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposal for the recognition of minimally differentiated acute myeloid leukaemia (AML-MO). Br J Haematol. 1991; 78: 325-329.
- WHO.WHO Handbook for reporting results of cancer treatment Geneva: World Health Organization, 1979.
- Hiddemann W, Clarkson BD, Buchner T, Melamed MR, Andreeff M. Bone marrow cell count per cubic millimeter bone marrow: a new parameter for quantitating therapy-induced cytoreduction in acute leukemia. Blood. 1982; 59: 216-225.
- Sakamoto Y, Uzuka Y, Saito Y. Strategy for the treatment of adult AML based on assessment of MRD. Int J Hematol. 2001; 73: 54.
- Kanai H, Koiwa Y, Saito Y, Susukida Y, Tanaka M. In vivo measurement of small velocity signal and change in thickness of the heart walls. Jpn J Applied Physics. 1999; 38: 6.
- Saito Y, Susukida I, Uzuka Y, Kanai H. Noninvasive early detection of anthracycline-induced cardiotoxicity in patients with hematologic malignancies using the phased tracking method. Cancer Med. 2016; 5: 2276-2285.
- Wheatley K, Brookes CL, Howman AJ, Goldstone AH, Milligan DW, Prentice AG, et al. Prognostic factor analysis of the survival of elderly patients with AML in the MRC AML11 and LRF AML14 trials. Br J Haematol. 2009; 145: 598-605.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987; 40: 373-383.
- Uzuka Y, Liong SK, Yamagata S. Treatment of adult acute non-lymphoblastic leukemia using intermittent combination chemotherapy with daunomycin, cytosine arabinoside, 6-mercaptopurine and prednisolone-DCMP two step therapy. Tohoku J Exp Med. 1976; 118: 217-225.
- Peterson BA, Bloomfield CD. Long-term disease-free survival in acute nonlymphocytic leukemia. Blood. 1981; 57: 1144-1147.
- Buchner T, Hiddemann W, Berdel W, et al. Remission induction therapy: the more intensive the better? Cancer Chemother Pharmacol. 2001; 48: S41-S44.
- Uzuka Y, Saito Y. Intensive induction therapy and postremission chemotherapy in adult acute nonlymphoblastic leukemia. Amsterdam-Priceton-Hong Kong-Tokyo-Sydney. Excerpta Medica press. 1987.
- Dykstra B, de Haan G. Hematopoietic stem cell aging and self-renewal. Cell Tissue Res. 2008; 331: 91-101.
- Haferlach T, Kern W, Schoch C, Schnittger S, Sauerland MC, Heinecke A, et al. A new prognostic score for patients with acute myeloid leukemia based on cytogenetics and early blast clearance in trials of the German AML Cooperative Group. Haematologica. 2004; 89: 408-418.
- Wheatley K, Burnett AK, Goldstone AH, Gray RG, Hann IM, Harrison CJ, et al. A simple, robust, validated and highly predictive index for the determination of risk-directed therapy in acute myeloid leukaemia derived from the MRC AML 10 trial. United Kingdom Medical Research Council's Adult and Childhood Leukaemia Working Parties. Br J Haematol. 1999; 107: 69-79.
- Estey EH, Shen Y, Thall PF. Effect of time to complete remission on subsequent survival and disease-free survival time in AML, RAEB-t, and RAEB. Blood. 2000; 95: 72-77.
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978; 4: 7-25.
- Xiao Z, Xue H, Li R, Zhang L, Yu M, Hao Y. The prognostic significance of leukemic cells clearance kinetics evaluation during the initial course of induction therapy with HAD (homoharringtonine, cytosine arabinoside, daunorubicin) in patients with de novo acute myeloid leukemia. Am J Hematol. 2008; 83: 203-205.
- Pettit K, Stock W, Walter RB. Incorporating measurable ('minimal') residual disease-directed treatment strategies to optimize outcomes in adults with acute myeloid leukemia. Leuk Lymphoma. 2016; 57: 1527-1533.