Research Article
Role of 18F-FDG PET/CT in Gastric Carcinomas: Comparison with Contrastenhancement Computed Tomography
Gonca Kara Gedik1*, Farise Yılmaz2, Mustafa Koplay2 and Oktay Sari1
1Department of Nuclear Medicine, Selçuk University, Turkey
2Department of Radiology, Selcuk University Faculty, Turkey
*Corresponding author: Gonca Kara Gedik, Department of Nuclear Medicine, Selcuk University, Turkey
Published: 17 Mar, 2017
Cite this article as: Gedik GK, Yılmaz F, Koplay M,
Sari O. Role of 18F-FDG PET/CT in
Gastric Carcinomas: Comparison with
Contrastenhancement Computed
Tomography. Clin Oncol. 2017; 2: 1221.
Abstract
Purpose: The purpose of this study was to investigate the usefulness of 18Fluorodeoxyglucose
Positron Emission Tomography /Computed Tomography (18F-FDG PET/CT) in patients with
gastric carcinoma and to compare it with contrast enhancement CT.
Materials and Methods: The files of 20 patients (5 female, 15 male, mean age: 68 years) who
underwent both 18F-FDG PET/CT and contrast enhancement CT for staging or restaging of gastric
carcinoma were retrospectively evaluated. For each modality, the sensitivity, specificity, positive
(PPV) and Negative Predictive Values (NPV) and accuracy was calculated in terms of gastric
lesion detection, lymph node metastases or distant metastases. The diagnostic performances of two
techniques were compared.
Results: For detecting gastric lesion, the sensitivity, specificity, PPV, NPV and accuracies of
18F-FDG PET/CT and contrast enhancement CT were 86%, 100%, 100%, 91%, 94% and 100%, 80%,
78%, 100% and 88%, respectively. In terms of lymph node metastases, the results were 75%, 71%,
60%, 83%, %73 and 88%, 92%, 88%, 92%, 90%, respectively. About distant metastases, the results
were, 64%, 89%, 90%, 62%, 74% and 64%, 44%, 64%, 44%, 57%, respectively. The differences of the
sensitivity and specificity of gastric lesion and metastatic lymph node detection were not statistically
significant between two imaging techniques (p >0.05, McNemar test). 18F-FDG PET/CT appeared
as a more specific modality than contrast enhancement CT for evaluating distant metastases in
patients with gastric carcinoma but difference could not be found as statistically significant (p >0.05,
McNemar test).
Conclusion: 18F-FDG PET/CT is a useful diagnostic modality for the evaluation of gastric lesion and
lymph node metastases and it has a comparable diagnostic performance with the standard imaging
procedure of contrast enhancement CT in patients with gastric carcinoma. For distant metastases,
complimentary usage of these techniques may be more beneficial in evaluation of patients with
gastric carcinoma.
Keywords: Gastric carcinoma; Positron emission tomography/computed tomography; Contrast enhancement computed tomography
Introduction
Gastric cancer is the 4th most common cancer worldwide and its mortality rate is second
to lung carcinoma [1]. Surgery has a curative potential and is the treatment of choice for early
gastric cancer. The primary aim of surgery is to eliminate the malignant tumor by resection of the
stomach and proper lymphadenectomy [2]. Although markedly reduced in recent years, surgical
morbidity from gastrectomy is still significant so preoperative determination of local extent of
disease and distant metastases is essential for planning the patient management. In order to avoid
unnecessary surgical procedures patients with advanced disease, who will not benefit from surgery
but must be referred combined chemotherapy and radiotherapy, must be carefully selected. This
question, whether the disease is localized or metastatic, can be answered by preoperative imaging
studies including endoscopic ultrasonography, contrast enhancement computed tomography and
occasionally laparoscopy.
With the recent technical advances in Computed Tomography (CT) and with the development of helical and multislice scanning, CT stands up as the primary
staging modality in gastric carcinoma. However, CT is an anatomybased
diagnostic technique which causes false negative results in
normal sized invaded lymph nodes and false positive in enlarged
inflammatory lymph nodes [3]. Like CT, especially for lymph node
staging, other morphology-based imaging tool such as endoscopic
ultrasonography and magnetic resonance imaging are also reported
as insufficient to guide therapeutic plans [4].
Positron Emission Tomography (PET) uses the radiolabeled
glucose analogue 18Fluorodeoxyglucose (18F- FDG), which shows
altered glucose metabolism in malignant tissue. PET imaging can
be combined with anatomic imaging such as CT which enables the
system to provide information about the anatomic localization of
abnormal foci of FDG uptake. After the introduction of combined
PET/CT modality into clinical practice in oncology environment,
this imaging system has been rapidly adopted in awide spectrumof
indications. In gastric carcinoma, preliminary results suggest that
it is of greater value in recurrent disease rather than for pretherapy
staging, but the benefits of PET/CT still remain uncertain [5].
The aim of this study is to evaluate the potential role of 18F-FDG
PET/CT in gastric cancer and to compare the diagnostic accuracy of
18F- FDG PET/CT with contrast enhanced CT for detection of gastric
lesion, lymph node and distant metastases.
Materıals and Methods
Patients
18F- FDG PET/CT scans of patients with gastric carcinoma
performed for staging or restaging in Selcuk University Faculty
of Medicine Department of Nuclear Medicine, between October
2012 and September 2015 were retrospectively reviewed. The ethics
committee of our institution approved this study (meeting date:
16.02.2016, no: 2016/3) and written informed consent was obtained
from all patients.
For inclusion the study, the required patient characteristics
were as followed: male or female patients with histopathologically
confirmed gastric carcinoma who underwent 18F- FDG PET/CT
scan and contrast enhancement CT within 30 days. Patients with
non epithelial gastric tumors, patients who either did not have or
who underwent contrast enhancement CT more than 30 days within
18F-FDG PET/CT and patients lost to follow-up, were excluded from
the study. Ultimately, the study included 20 patients (5 female, 15
male, age range: 36-81 years, mean: 68 years).
18F-FDG PET/CT technique
Images from the skull base to the mid thigh were acquired in 8 or
9 bed positions with an acquisition time of 2 minutes per bed position
with integrated 18F- FDG PET/CT scanner (Biographm CT, Siemens,
Germany). Patients were advised to fast for at least 6 hours and 370
Mega Becquerel (MBq) of 18F-FDG was injected to the patients whose
blood glucose level was <200 mg/dl. PET/CT studies were performed
1 hour after the administration of the radiopharmaceutical. After
radiopharmaceutical injection, 1000 ml water without contrast
material was also applied orally. The CT part of the integrated scan
was carried out without contrast enhancement by using 16 slice CT
with the acquisition parameters of 190 mA, 5 mm slice thickness and
140 kV. Right after the CT imaging, PET scan was performed without
changing the position. The CT data were used for the attenuation
correction of PET scanning.
Contrast enhancement CT technique
Contrast enhancement CT examinations were performed with a
dual-source 128 x 2-slice DSCT scanner (Somatom Definition Flash,
Siemens Healthcare Forscheim, Germany) which has two X-ray tubes
located at 95º angle and 128-channel two-detector row. The acquisition
parameters were as follows: slice thickness 3 mm, spiral pitch factor
0.6, gantry rotation time 280 ms, kVp 120, 180-420 mAs. All scans
included oral and intravenous contrast enhanced administration.
Water was also orally applied prior to CT examination in order to
provide gastric distention. IV contrast medium (iohexol, iodine
content 350 mg/mL; Omnipaque, GE Health care) was administered
from brachial vein at a flow rate of 2-3 mL/s. In order to reduce the
artifact of contrast medium, a saline solution (40 ml) was injected.
F-FDG PET/CT and contrast enhancement CT interpretations
18F-FDG PET/CT and contrast enhanced CT examinations were
reviewed by 2 nuclear medicine physicians and by 1 radiologist,
respectively. All of the reviewers were experienced and unaware of
the patient medical history. The images of 18F- FDG PET/CT and
contrast enhancement CT were reviewed for evidence of primary
tumor / locally recurrent disease, lymph node and distant metastases.
Acquired images of 18F-FDG PET/CT were analysed on Siemens
Syngo.via PET-CT workstation. 18F-FDG PET/CT was considered
as positive for primary tumor if any increased FDG uptake greater
than the adjacent normal gastric wall. Focal or diffuse increased FDG
uptake in postsurgical area was accepted as recurrent disease. FDG
uptake of lymph node was considered as malignant if fatty hilum was
not observed. For mediastinum, lymph nodes showing FDG uptake
higher than mediastinal vascular structures, interpreted as metastatic.
Any lesion in sites different from the stomach or lymph nodes showing
FDG accumulation regarded as distant metastases. Volume of interest
was drawn on the high FDG uptake area and maximum Standardized
Uptake Value (SUVmax), which is a semiquantitativeparameter for
the FDG uptake normalized to the injected dose and patient weight,
was calculated for each gastric lesion of showing FDG uptake.
For contrast enhancement CT, a gastric lesion was considered
to be cancerous when a polipoid mass or gastric wall thickening of
>3 mm was observed. Lymph nodes were described as metastatic if
they were larger than 10 mm in the short-axis diameter. If there was
at least one lesion in regions different from the stomach and lymph
nodes, it was regarded as distant metastases.
In the next step, disease involved areas for thorasic and abdominal
parts were introduced for metastatic lymph node and distant
metastatic areas. Each patient took a score according to the number of
disease involved areas for each imaging modality. In thorasic region,
mediastinum, parasternal, both axillas, and right and left lung were
determined as lymph node and distant metastatic areas, respectively. If
a patient had metastatic noduler lesions in right and left lungs, he took
a score of 2 for distant metastasis for thorasic region. If mediastinal
lymph nodes were involved with metastatic disease, the patient took
score 1 for lymph node metastases for thorasic region regardless
the number of involved stations. In abdominal region, perigastric,
paraaortic-paracaval, right and left inguinal and mesenteric lymph
node areas were determined as disease involved areas for lymph node
metastases. Periton, liver, right and left adrenal glands and spleen
were determined as distant metastatic areas for abdomen. For each
area, the patient took score of 1. If a patient had bone involvement, he took a score of 1 for distant metastases, regardless of the number of involved bones.
Gold standard for comparison the results of contrast enhancement
CT and 18F-FDG PET/CT was accepted as surgical intervention/
biopsy (n:8 patients) or clinical-instrumental follow-up of at least 6
months (contrast enhancement CT, 18F-FDG PET/CT or magnetic
resonance imaging, n: 12 patients).When only one regional CT
was present (thorax or abdomen), only that region was taken into
consideration during comparison of 18F-FDG PET/CT and contrast
enhancement CT. For both imaging modalities; any reported disease
involved area, proved as gastric pathology, lymph node metastases
or distant metastases by follow-up studies or by surgery, has been
classified as true positive. If an area was reported as disease involved
but turned out to be reactive by biopsy or no more observed in followup
studies without taking chemo/ radiotherapy, it was classified as
false positive. An area which was not reported as disease involved by
one of the modality but as involved by other and proved as gastric
lesion, lymph node or distant metastases, by pathology or in followup
studies, false negativity was assigned for the former technique. If
an area was reported as free of disease by one of the modality but
as metastatic by the other one, and biopsy or follow-up clarified the
absence of disease, true negativity was concerned for the former
modality.
Statistical analysis
Sensitivity, specificity, negative and positive predictive values
and accuracy of 18F-FDG PET/CT and contrast enhancement CT
were calculated for gastric lesion, lymph node and distant metastases.
For lymph node and distant metastases, area based analysis was
performed. The diagnostic performance of two modalities was
compared by McNemar test. Statistical significance was assumed
when a p value was less than 0.05.
Table 1
Table 1
Clinical and pathological characteristics of patients, results of 18F-FDG PET/CTand contrast enhancement CT.
Abbrevations: AC: Adenocarcinoma; MAC: Mucinous Adenocarcinoma; SRCC: Signet Ring Cell Carcinoma; PDCH: Poorly Differentiated Cohesive Carcinoma;
M: Male, FM: Female; GL: Gastric Lesion; LNM: Lymph Node Metastases; DM: Distant Metastases; CECT: Contrast Enhancement CT; *: Total Number Of Disease
Involved Areas Proved By Gold Standard; -: Not Evaluated in Terms of Gold Standard; not available: Patients with Only Thoraks CT
Results
In 14 patients 18F-FDG PET/CT was performed for restaging and
in 6 for staging. Ten patients underwent both thorax and abdomen
contrast enhancement CT and 18F-FDG PET/CT and in remaining 10,
only one regional contrast enhancement CT was present. The mean
time interval between 18F-FDG PET/CT and contrast enhancement
CT was 12 days (range: 2-30 days). Clinical and pathological
characteristics of patients and the results of both imaging modalities
are shown in Table 1.
Gastric lesions
Totally, 7 gastric lesions were clarified as primary diagnosis/
tumor recurrence with gold standard in 20 patients.18F-FDG PET/
CT resulted positive for gastric lesions in all of 6 of 7 lesions (86%,
Figure 1). Mean SUVmax was calculated as 17.40 (range: 3.67-35.30).
In 1 patient who was submitted forstaging, 18F-FDG PET/CT failed to
show increased activity in gastric lesion. Contrast enhancement CT
detected all gastric lesions (100%). There were no false negative results
and 2 lesions were falsely reported as positive for gastric lesion with
contrast enhancement CT. Results concerning sensitivity, specificity,
PPV, NPV and accuracy are depicted in Table 2 and the differences
of sensitivity and specificity were not statistically significant between
two imaging modalities (p = 1.00 and p = 0.5, respectively, McNemar test).
Lymph node metastases
Eight metastatic lymph node areas were clarified in 20 patients.
18F-FDG PET/CT resulted positive for metastases in10 lymph node
areas. Among these, 6 of them were true and 4 were false positive
areas. Two metastatic lymph node areas were missed in 2patients
with 18F-FDG PET/CT (patients no:11 and 20, Table 1and Figure
2). Contrast enhancement CT resulted positive for lymph node
involvement in 8 lymph node areas. Onefalse positive (patient no: 4, Table 1) and 7 true positive areas were noted with contrast
enhancement CT. One false negative area in 1 patient (patient no:9,
Table 1 and Figure 3) was also recognized. Contrast enhancement CT
was found more sensitive and specific than 18F-FDG PET/CT(88%
vs. 75% and 92% vs. 71%, respectively; Table 2) but the differences
were not statistically significant (p = 1.00 and p = 0.375, respectively,
McNemar test).
Distant metastases
Totally 14 distant metastatic areas were identified in 20 patients.
Among these 14 distant metastatic areas,18F-FDG PET/CT correctly
detected 9 of them and 5areas were missed with this modality. One false positive are was noted with 18F-FDG PET/CT (patient no: 7,
Table 1). Similarly, contrast enhancement CT, resulted in 9 true
positive and 5 false negative areas. There were 5 areas which were
reported as false positive with, contrast enhancement CT and in 1
patient, metastatic bone lesions were only detected with 18F-FDG
PET/CT but were missed with contrast enhancement CT(patient no:
13, Table 1and Figure 4). In 1 patient bilaterally located metastatic
surrenal lesions were falsely reported as adenoma with both
imaging modalities (patient no: 20, Table 1). Peritoneal seeding
was missed in 2 patients with 18F-FDG PET/CT but detected with
contrast enhancement CT (patients no: 2 and 15, Table 1). Omental
metastases in 1 patient was correctly detected with 18F-FDG PET/
CT but was missed with contrast enhancement CT (patient no: 17,
Table 1).The sensitivity of both imaging modalities were calculated
as 64%, the specificity of 18F-FDG PET/CT was higher than contrast
enhancement CT but the difference was not statistically significant (p
= 0.125, McNemar test, Table 2).
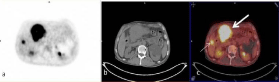
Figure 1
Figure 1
Transaxial PET (a), CT (b) and fusion (c) images of 18F-FDG PET/
CT Study Showed primary gastric tumor located in antrum (thick arrow, c)
and metastatic foci of FDG uptake in liver (thin arrow, c).
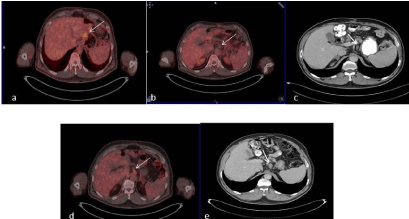
Figure 2
Figure 2
Fifty-five years old male patient was submitted for restaging of
gastric carcinoma. Transaxial fusion image of 18F-FDG PET/CT demonstrated
the recurrent disease postsurgical area of showing FDG uptake (a, arrow). b:
Transaxial fusion image of 18F-FDG PET/CT from lower part. Metastatic lymph
node without FDG uptake was not regarded as disease involved (arrow).
With contrast enhancement CT, perigastric lymph node was reported as
metastatic (c, arrow). In follow-up studies, high FDG uptake (e, arrow) and
enlargement (f, arrow) of metastatic perigastric lymph node was recognized
with 18F-FDG PET/CT and contrast enhancement CT, respectively.
Table 2
Table 2
Results concerning the sensitivity, specificity, accuracy, PPV and NPV of both imaging modalities.
Abbrevations: GL: Gastric Lesion; LNM: lymph Node Metastases; DM: Distant Metastases; PPV: Positive Predictive Value; NPV: Negative Predictive Value; CECT:
Contrast Enhancement CT
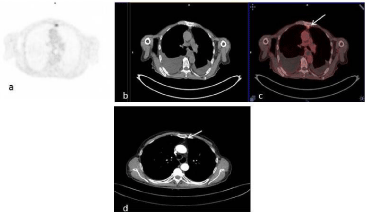
Figure 3
Figure 3
Transaxial PET (a), CT (b) and fusion (c) images of 18F-FDG
PET/CT. Metastatic left parasternal lymph node showing FDG uptake was
reported as disease involved (c, arrow). Same lymph node measuring 8
millimeters short-axis diameter, was not recognized as metastatic with
contrast enhancement CT (d, arrow).
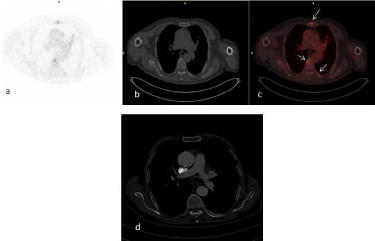
Figure 4
Figure 4
Transaxial PET (a), CT (b) and fusion (c) images of 18F-FDG PET/
CT study of 81 years-old male patient. Metastatic bone lesions in sternum
and thoracal vertebra showing FDG uptake were observed with 18F-FDG
PET/CT (arrows, c). Contrast enhancement CT missed these metastatic
deposits in bones (d).
Discussion
18F-FDG PET/CT has emerged to provide increased accuracy
compared with the conventional tools for the diagnosis of primary
and recurrent gastrointestinal tumors including esophageal and
colorectal cancer [6]. In gastric carcinoma, correlation of primary
tumor of FDG uptake with histopathological features of gastric
cancer has been investigated and 18F-FDG PET/CT is reported to
have limited role in primary diagnosis because of relatively high
number of primary tumors that are not avid for 18F-FDG such as
signet ring cell carcinoma and cancer with high mucinous content
[7,8]. For the detection of recurrent gastric cancer; in some studies
it is reported that FDG-PET has a low diagnostic accuracy [9].
On the other hand by Bilici et al. [10] 18F-FDG PET/CT has been
reported as a superior post-therapy surveillance modality for the
diagnosis of recurrent gastric cancer compared with diagnostic CT.
In our study, 6 of 7 (86%) gastric lesions were correctly detected
with 18F-FDG PET/CT. For detecting gastric tumors, sensitivity of
21% to 100% has been reported in the literature [11]. In recurrent
gastric carcinoma, sensitivity values such as 78% and 75 % have been
reported for 18F-FDG PET/CT [12,13]. High sensitivity results of
86% for detecting gastric lesion was reached in our study. Moreover,
high SUVmax of 9.29 was calculated in one of the patients with
signet cell carcinoma which is discordant with the reported low
sensitivity in this type of cancer due to extracellular or intracellular
mucin component. Our high sensitivity results can be attributed
to preparation of patients before 18F-FDG PET/CT examination. It
has been shown that distension of stomach using water improves
the diagnostic performance of 18F-FDG PET/CT in detecting both
primary and recurrent gastric tumors [14,15]. In our department
we use water prior to PET/CT acquisition to achieve this distension
which may have an effect on value of our sensitivities. The localization
of tumor has also been discussed that may affect the detection of
gastric carcinomas and gastro esophageal junction carcinomas was
reported to have a higher sensitivity than other stomach parts [16].
In our study, 3 of 7 lesions were located in corpus, 3 including the
one which 18F-FDG PET/CTmissed in antrumand 1 was in cardia
which supported the independency of gastric carcinoma detectability
by site like other studies [17]. The data about sizes of the tumors was
not available in our study so the effect of size of the tumor on lesion
detectability could not be drawn. Except 2, all the patients showing
FDG uptake, had a diagnosis of gastric adenocarcinoma, so the
differences of SUVmax values between different histologic subtypes
could not be assessed. The patient missed with 18F-FDG PET/CT but
correctly diagnosed with contrast enhancement CT was a patient
with gastric adenocarcinoma and we thought that physiologic gastric
FDG uptake in gastric smooth muscle adjacent to the primary tumor
obscured the lesion. Coexisting inflammatory changes may also
play role in accumulating 18F-FDG in gastric mucosa. On the other
hand, contrast enhancement CT correctly detected all the primary
and recurrent tumors. Since contrast enhancement CT is based on
size dependent interpretation criteria, areas showing wall thickening
but free of disease, 2 patients were falsely reported to have tumor
recurrence by contrast enhancement CT which reduced the specificity
of this technique. We thought that morphologic changes evolved after
surgery and deteriorated anatomy, caused these false positive results.
Although the differences were not statistically significant, in terms
of detecting gastric lesions, 18F-FDG PET/CT was found to be more
specific (100% vs. 80%) but less sensitive (86% vs. 100%) than contrast
enhancement CT in our study which was concordant with the results
of Altini et al [11].
The presence of lymph node metastases is an important prognostic
factor in gastric carcinoma. 18F-PET/CT assesses metabolic and
functional aspects better than the anatomic features of the tumor
so occult metastases can be better detected than the conventional
anatomy based imaging techniques. However, 18F-FDG is not specific
for neoplastic cells and inflammatory tissue may also show intense
18F-FDG uptake. In our study, 1 patient in whom FDG uptake of
right paratracheal lymph node was observed, but clarified as reactive
in follow-up, was falsely classified as metastatic. Conversely, another
patient with metastatic lymph nodes located in lung hilum were
missed by 18F-FDG PET/CT because of physiological FDG uptake
in lung hilum which is a frequent finding in18F-FDG PET/CT but
correctly diagnosed by CT. Another patient was a 55 year-old male
with metastatic lymph node in perigastric area who was presented
with recurrent disease. In this patient recurrent disease could be
correctly detected with 18F-FDG PET/CT but metastatic perigastric
lymph node was missed. A known limitation of 18F-FDG PET/CT
systems is about their spatial resolution which prevents discriminating
perigastric lymph nodes from primary tumors. However, in our
patient the reason of false negativity was the low FDG uptake of
metastatic lymph node. One parasternal metastatic lymph node area
showing FDG uptake was missed with contrast enhancement CT
but was caught by18F-FDG PET/CT because enlargement of lymph
nodes was not observed in disease involved lymph node on contrast
enhancement CT.
In our study, the sensitivity of 18F-FDG PET/CT was found
higher than the results of Altini et al. [11] Ha et al. [18] and Kwee et
al. [19]. The diagnostic performance of 18F-FDG PET for detecting
lymph node involvement depends on many factors including the
avidity of primary tumor for 18F-FDG. In our study group, 18 of 20
(90%) patients had a diagnosis of nonmucinous type adenocarcinoma
which has been reported to be as 18F-FDG avid.
The trend of higher sensitivity of contrast enhancement
CT than18F-FDG PET/CT for lymph node metastases in gastric
carcinoma reported in the literature was also obvious in our study.
Specificity, PPV and NPV were also higher for contrast enhancement
CT than 18F-FDG PET/CT. Taken together, contrast enhancement
CT was more accurate than 18F-FDG PET/CT for evaluating lymph
node metastases.
In evaluating distant metastases, same results appeared for both modalities in terms of sensitivity (64%). Sensitivity values in gastric
carcinoma for distant metastases reported with 18F-FDG PET/
CT changes in the literature. For detecting solid organ metastases
sensitivity of 95.2% was reported by Chung et al. [20] for 18F-FDG
PET/CT. However, reported a value of 60% for distant metastases
evaluation.18F-FDG PET/CT displays functional aspects of tumor and
metabolic abnormalities precede the morphologic changes shown
by anatomic imaging like CT. This drawback of anatomic imaging
recognized also in our study and metastatic bone deposits that were
hidden in well preserved anatomic structures, could only be detected
with 18F-FDG PET/CT, which was discordant with the results of
Yoshioka et al [21]. Like reported in the literature for evaluating
peritoneal dissemination, low sensitivity compared to contrast
enhancement CT was noted also in our study and 2 patients with
peritoneal implants could only be detected with contrast enhancement
CT [3,21]. The specificity of 18F-FDG PET/CT for detecting distant
metastases was higher than contrast enhancement CT but was not
statistically significant which can be attributed to the low number
of patients in each group. The higher specificity 18F-FDG PET/CT
than contrast enhancement CT for detecting distant metastases was
concordant with the literature [11] and the low specificity of contrast
enhancement CT can be attributed to size dependent interpretative
criteria of this imaging modality.
The limitation of our study was its retrospective origin. That’s
why small number of patients could be included to the study. Lack
of data about the size of primary tumor and histological confirmation
of lymph node or distant metastatic areas can also be counted as
limitations of our study.
In conclusion, our data showed that 18F-FDG PET/CT is a
useful diagnostic modality for the evaluation of gastric carcinoma in
detecting primary tumor, recurrent disease, lymph node and distant
metastases. It has a comparable diagnostic performance with the
standard imaging procedure of contrast enhancement CT in gastric
carcinoma. Its higher specificity may reduce futile laparotomies.
Because of the additive information provided by each modality,
complimentary usage of these techniques may be more beneficial in
evaluation of patients with gastric carcinoma.
References
- Ma Q, Xin J, Zhao Z, Guo Q, Yu S, Xu W, et al. Value of 18F-FDG PET/ CT in the diagnosis of primary gastric cancer via stomach distension. Eur J Radiol. 2013; 82: 302-306.
- Lee JW, Lee SM, Lee MS, Shin EC. Role of 18F-FDG PET/CT in the prediction of gastric cancer recurrence after curative surgical resection. Eur J Nucl Med Mol Imaging. 2012; 39: 1425-1434.
- Chen J, Cheong JH, Yun MJ, Kim J, Lim JS, Hyung WJ, et al. Improvement in preoperative staging of gastric adenocarcinoma with positron emission tomography. Cancer. 2005; 103: 2383-2390.
- Yun M, Lim JS, Noh SH, Hyung WJ, Cheong JH, Bong JK, et al. Lymph node staging of gastric cancer using (18)F-FDG PET: a comparison study with CT. J Nucl Med. 2005; 46: 1582-1588.
- Hur H, Kim SH, Kim W, Song KY, Park CH, Jeon HM, et al. The efficacy of preoperative PET/CT for prediction of curability in surgery for locally advanced gastric carcinoma. World J Surg Oncol. 2010; 8: 86.
- De Potter T, Flamen P, Van Cutsem E, Penninckx F, Filez L, Bormans G, et al. Whole-body PET with FDG for the diagnosis of recurrent gastric cancer. Eur J Nucl Med Mol Imaging. 2002; 29: 525-529.
- Kim H, Won KS, Song BI, Kang YN. Correlation of primary tumor FDG uptake with histopathologic features of advanced gastric cancer. Nucl Med Mol Imaging. 2015; 49: 135-142.
- Herrmann K, Ott K, Buck AK, Lordick F, Wilhelm D, Souvatzoglou M, et al. Imaging gastric cancer with PET and the radiotracers 18F-FLT and 18F-FDG: a comparative analysis. J Nucl Med. 2007; 48: 1945-1950.
- Choi BW, Zeon SK, Kim SH, Jo I, Kim HW, Won KS. Significance of SUV on follow-up F-18 FDG PET at the anastomotic site of gastroduodenostomy after distal subtotal gastrectomy in patients with gastric cancer. Nucl Med Mol Imaging. 2011; 45: 285-290.
- Bilici A, Ustaalioglu BB, Seker M, Kefeli U, Canpolat N, Tekinsoy B, et al. The role of 18F-FDG PET/CT in the assessment of suspected recurrent gastric cancer after initial surgical resection: can the results of FDG PET/ CT influence patients' treatment decision making? Eur J Nucl Med Mol Imaging. 2011; 38: 64-73.
- Altini C, NicolliAsabella A, Di Palo A, Fanelli M, Ferrari C, Moschetta M, et al. 18F-FDG PET/CT role in staging of gastriccarcinomas: comparison with conventional contrastenhancement computed tomography. Medicine. 2015; 94.
- Nakamoto Y, Togashi K, Kaneta T, Fukuda H, Nakajima K, Kitajima K, et al. Clinical value of whole-body FDG-PET for recurrent gastric cancer: a multicenter study. Jpn J ClinOncol. 2009; 39: 297-302.
- Park MJ, Lee WJ, Lim HK, Park KW, Choi JY, Kim BT. Detecting recurrence of gastric cancer :the value of FDG PET/CT. Abdom Imaging. 2009; 34: 441-447.
- Zhu Z, Li F, Zhuang H. Gastric distension by ingesting food is useful in the evaluation of primary gastric cancer by FDG PET. Clin Nucl Med. 2007; 32: 106-109.
- Yun M. Imaging of gastric cancer metabolism using 18 F-FDG PET/CT. J Gastric Cancer. 2014; 14: 1-6.
- Stahl A, Ott K, Weber WA, Becker K, Link T, Siewert JR, et al. FDG PET imaging of locally advanced gastric carcinomas: correlation with endoscopic and histopathological findings. Eur J Nucl Med Mol Imaging. 2003; 30: 288-295.
- Wu AJ, Goodman KA. Positron emission tomography imaging for gastroesophageal junction tumors. Semin Radiat Oncol. 2013; 23: 10-15.
- Ha TK, Choi YY, Song SY, Kwon SJ. F18-fluorodeoxyglucose-positron emission tomography and computed tomography is not accurate in preoperative staging of gastric cancer. J Korean Surg Soc. 2011; 81: 104- 110.
- Kwee RM, Kwee TC. Imaging in assessing lymph node status in gastric cancer. Gastric Cancer. 2009; 12: 6-22.
- Chung HW, Lee EJ, Cho YH, Yoon SY, So Y, Kim SY, et al. High FDG uptake in PET/CT predicts worse prognosis in patients with metastatic gastric adenocarcinoma. J Cancer Res ClinOncol. 2010; 136: 1929-1935.
- Yoshioka T, Yamaguchi K, Kubota K, Saginoya T, Yamazaki T, Ido T, et al. Evaluation of 18F-FDG PET in patients with advanced, metastatic, or recurrent gastric cancer. J Nucl Med. 2003; 44: 690-699.