Mini Review
Choline PET/CT Imaging for Management of Prostate Cancer
Kazuhiro Kitajima1*, Kazuhito Fukushima1, Koichiro Yamakado2, Shozo Hirota2 and Shingo Yamamoto3
1Department of Radiology, Division of Nuclear Medicine and PET Center, Hyogo College of Medicine, Japan
2Department of Radiology, Hyogo College of Medicine, Japan
3Department of Urology, Hyogo College of Medicine, Japan
*Corresponding author: Kazuhiro Kitajima, Department of Radiology, Division of Nuclear Medicine and PET center, Hyogo College of Medicine, Nishinomiya, 1-1Mukogawacho, Nishinomiya, Hyogo 663-8501, Japan
Published: 16 Mar, 2017
Cite this article as: Kitajima K, Fukushima K, Yamakado K,
Hirota S, Yamamoto S. Choline PET/CT
Imaging for Management of Prostate
Cancer. Clin Oncol. 2017; 2: 1218.
Abstract
Whole-body Positron Emission Tomography/Computed Tomography (PET/CT) with [11C]- and
[18F]-labeled choline derivatives has emerged as a promising molecular imaging modality for
evaluation of prostate cancer. 11C-choline and 18F-flurocholine PET/CT examinations have been
shown to be effective for restaging of prostate cancer patients with biochemical disease recurrence
after undergoing definitive therapy, especially those with a serum prostate-specific antigen level
>1.0 ng/mL. On the other hand, they have more limited roles for initial staging of prostate cancer
or detection of tiny lymph node metastasis, due to the low spatial resolution inherent with PET.
Overall, these modalities are most useful for cases with a high pre-test suspicion of metastatic
disease. Here, we review the current clinical roles of 11C-choline and 18F-fluorocholine PET/CT for
management of patients with prostate cancer.
Keywords: prostate cancer; Staging; Restaging; 11C-choline; 18F-fluorocholine; Positron emission tomography/computed tomography (PET/CT)
Introduction
Integrated Positron Emission Tomography/Computed Tomography (PET/CT) is a unique imaging technique for acquisition of both metabolic and anatomical imaging data using a single device in a single diagnostic session, which has opened new opportunities for clinical oncological imaging of various types of malignant tumors. The most commonly used radiopharmaceutical for PET in oncology cases is 2-[18F] fluoro-2-deoxyd-glucose (18F-FDG), an analog of glucose that is preferentially taken up by and trapped inside malignant cells. However, for urologic oncology, use of 18F-FDG is limited for diagnosis of localized prostate cancer, because of its low level of tumor uptake and urinary excretion [1,2]. In recent years, new and more promising PET tracers, such as 11C-acetate,11C-choline,18F-fluorocholine, anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid (anti-3-18F-FACBC), and Prostate-Specific Membrane Antigen (PSMA), have been evaluated for imaging of patients for prostate cancer detection [1-3]. Here, we review the current and future roles of choline PET/CT for management of prostate cancer, and also discuss its usefulness and limitations for imaging of affected patients.
Choline
The concept for using choline in prostate cancer imaging is based on elevated phosphorylcholine
levels and increased phospho-choline turnover in prostate cancer cells [4]. Choline is an essential
component of cell membrane phospholipids. After uptake into the cell through a high-affinity
transporter system, choline kinase phosphorylates choline, which represents the first step in the
Kennedy cycle, and is then incorporated into the phosphatidyl membrane. In prostate cancer cells,
key enzymes of choline metabolism, such as cholinekinase, are upregulated.
Hara et al. [5] were the first to describe use of 11C-choline PET for evaluation of prostate cancer.
A major advantage of this radiotracer is its rapid blood clearance (~5 minutes) and rapid uptake by
prostate tissue (3–5 minutes), which allows for early imaging prior to excretion of the radiotracer
into urine. Thus, the pelvis can be viewed before significant excretory activity becomes a potential
confounder. Unfortunately, the 20-minute half-life of 11C-choline restricts its use to institutions
equipped with a cyclotron on site, whereas the longer half-life of 18F-flurocholine (~110 minutes)
allows transportation from institutions without a cyclotron. In addition, the shorter positron range of 18F-fluorocholine over 11C-choline produces slightly higher image
quality, though the urinary excretion of 18F-flurocholine is greater
than that of 11C-choline [6].
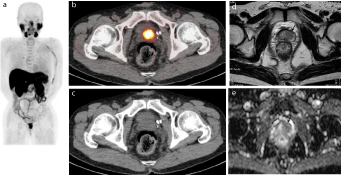
Figure 1
Figure 1
Representative case of 63-year-old male(PSA 5.05 ng/mL) who underwent androgen-deprivation therapy for prostate cancer.
(a) MIP of 11C-choline PET showing abnormal choline uptake in prostate.
(b) 11C-choline PET/CT and (c) CT portion of PET/CT showing intense FDG uptake in left side of prostate, suggesting local recurrence.
(d) T2-weighted MR image showing slightly hypointense mass in left transition zone of prostate (arrow), consistent with local recurrent prostate cancer.
(e) Apparent Diffusion Coefficient (ADC) map of diffusion weighted magnetic resonance imaging showing a hypointense area in the same area shown in (d) (arrow).
Diagnosis
Focal choline uptake by the prostate leads to suspicion of prostate cancer. However, non-malignant causes, such as high-grade prostatic intraepithelial neoplasia, prostatitis, Benign Prostatic Hyperplasia (BPH), and even normal tissues can also be sources of false-positive focal activity. In patient-based analyses, detection of local untreated prostate cancer by choline PET/CT has been reported to have a sensitivity of 55–100%, specificity of 43–87%, and accuracy of 60–84 % [1,3]. Sensitivity is related to lesion size and Martorana et al. [7] found that sensitivity in 11C-choline PET/CT examinationswas83% for lesions ≥5 mm, while it was only 4% for lesions <5 mm. This is not surprisingly, since the spatial resolution of clinical PET scanners is about 5 mm. In addition, a partial volume effect could be another cause of failure to detect smaller lesions [8]. Although a few groups have shown that increased choline uptake in primary prostate cancer is correlated with histological surrogate markers of aggressiveness, such as Gleason score and MIB-1/Ki- 67 labeling index [9], many groups have failed to find a significant correlation between choline uptake and serum Prostate Specific Antigen (PSA) level, Gleason score, or tumor grade [10,11]. Multiparametric Magnetic Resonance Imaging (MRI), a combination of high-resolution T2-weighted imaging and functional MR techniques such as dynamic contrast-enhanced imaging and diffusion-weighted imaging, remains the gold standard imaging technique for detection and local staging of untreated prostate cancer, as it shows such details as capsular and seminal vesicle invasion.
Staging
Although choline PET/CT has limitations for detection of primary prostate cancer, it may be useful for a minority of newly diagnosed patients in whom distant metastatic disease is highly suspected on the basis of clinical data (e.g., serum PSA level >20 ng/ml, Gleason score 8–10, locally advanced tumor evident by palpation and/or MRI) [1-3]. Beheshti et al. [11] evaluated 18F-fluorocholine PET/CT for pre-treatment staging of prostate cancer in 130 intermediateand high-risk patients, and concluded that results obtained with18Ffluorocholine PET/CT would have prompted a change of therapy in 15% of all patients and 20% of the high-risk patients. Although choline PET/CT shows better performance than conventional CT or MR imaging for detection of Loco-Regional Lymph Node (LN) metastasis, it does not reach an optimal detection rate in comparison with a lymphadenectomy, thus it is not expected to replace that procedure. In the largest reported preoperative series (n = 210) of intermediate- and high-risk patients who had underwent a Radical Prostatectomy (RP) with surgical LN dissection, Poulsen et al. [12] reported that patient-based sensitivity and specificity for detection of pelvic LN metastasis by 18F-fluorocholine PET/CT were 73% and 88%, respectively, with node-based sensitivity and specificity of 56% and 94%, respectively. The sensitivity of choline PET/CT for nodal prostate cancer metastasis appears to be related to the size of the pathologic node. Two other groups reported a 0% detection rate for nodes <2 mm in size, while that was 25–30% for nodes sized 2–4.9 mm, 33–43% detection for nodes sized 5–9.9 mm, and 77–90 % for nodes measuring at least 10 mm [13,14]. Choline PET/CT has been shown to be a useful modality for detection of bone metastasis. Picchio et al. [15] directly compared 11C-choline PET/CT and bone scintigraphy for detection of bone metastasis in 78 patients with PSA progression after primary treatment, and demonstrated that 11C-choline PET/CT exhibited an equivalent sensitivity but higher specificity. In their study, patientbased sensitivity, specificity, and accuracy of 11C-choline PET/CT were 89%, 98–100%, and 95–96%, respectively, while those for bone scintigraphy were 70–100%, 75–100%, and 83–90%, respectively. Occasionally, osteoblastic lesions identified by bone scintigraphy show no choline uptake (false-negative PET result) [15,16], thus it is important to note any osteoblastic change in the CT portion of a PET/CT examination. Beheshti et al. [17] reported sensitivity, specificity, and accuracy of 79%, 97%, and 84% for detection of bone metastasis using 18F-fluorocholine PET/CT in 70 patients who were undergoing either initial staging or restaging. They also noted that choline activity tended to vary inversely with the degree of lesion sclerosis, as the maximum standard uptake value (SUVmax) for lytic lesions was 11±3.2 and that for sclerotic lesions was 7.8±3.0. Lesions with dense sclerosis shown by CT (>825 Hounsfield units) had no choline activity and were mainly observed in patients being treated with hormone therapy, suggesting healed bone metastasis.
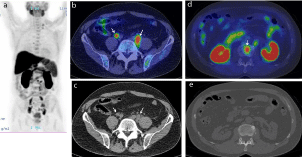
Figure 2
Figure 2
Representative case of 67-year-old male(PSA 9.87 ng/mL) who underwent external beam radiation therapy.
(a) MIP of 11C-choline PET showing abnormal choline uptake in left supraclavicular, mediastinal, abdominal, and pelvic areas.
(b) 11C-choline PET/CT and (c) CT portion of PET/CT showing intense FDG uptake corresponding to mild-swollen common iliac lymph nodes (arrows), suggesting
lymph node metastasis. Detection of these tiny nodal metastases by only CT (c) would be difficult.
(d) 11C-choline PET/CT and (e) CT portion of PET/CT showing intense FDG uptake corresponding to lumber vertebra, suggesting bone metastasis. Detection of
these tiny bone metastases by only CT (e) would be difficult.
Restaging
In patients with biochemical failure, imaging plays a critical role in distinguishing between local recurrence and distant spread of disease (mostly bone and LN metastasis) when formulating an appropriate treatment strategy. Choline PET/CT is a powerful tool for restaging of biochemically recurrent prostate cancer, particularly in patients with a significantly elevated PSA level. Many reports have discussed the usefulness of choline PET/CT for detecting sites of recurrence in patients with PSA failure (Figure 1 and 2), and choline PET/CT is routinely employed for this purpose at a large number of PET centers in Western countries. Giovacchini et al. evaluated the findings of [11C]-choline PET/CT in 358 patients (PSA 0.23–45.0 ng/ ml, median1.27 ng/ml) treated with RP and reported that patientbased sensitivity, specificity, and accuracy for restaging of prostate cancer was85%, 93%, and 89%, respectively, while the percentage of positive scan findings was 19% in patients with a PSA level of 0.23–1 ng/ml, 46% in those with PSA of 1–3 ng/ml, and 82% in those with PSA>3 ng/ml [18]. In a multivariate analysis of 1000 patients with biochemical evidence of recurrence after various treatments (PSA 1.15–11.0 ng/ml, median 3.30 ng/ml), Cimitan et al. [19] revealed that only older age, Gleason score ≥7, systemic chemotherapy, and serum PSA level ≥1 ng/ml were independent predictors of 18F-choline PET/CT positivity. In a systematic review that included meta-analysis of 19 selected studies with a total of 1555 patients revealed a pooled sensitivity of 85.6% and pooled specificity of 92.6 % [20]. In recent years, two other studies revealed that multi-parametric MRI with an endorectal coil is superior for detection of local recurrence after RP relative to choline PET/CT [21,22], indicating that, strictly speaking, the combination of multi-parametric MRI and choline PET/CT should be an ideal tool for restaging of patients with PSA failure. In the future, wider application of integrated PET/MRI can be expected.
PET/MRI
In recent years, interest has been increasing in development of integrated PET/MRI systems, which have become commercially available. PET/MRI has a number of advantages over PET/CT, such as improved soft-tissue contrast, possibility of performing truly simultaneous instead of sequential acquisitions, and availability of sophisticated MRI sequences, such as diffusion and perfusion imaging, as well as functional MRI and MR spectroscopy, which can add important information. Moreover, a significant decrease in radiation exposure is seen with PET/MRI, which is of foremost importance for serial follow-up imaging examinations and in pediatric cases. In addition, several groups have demonstrated the usefulness of PET/ MRI for patients with prostate cancer using choline [23,24] or PSMA [25].
Conclusion
Choline PET/CT has been successfully used for restaging of prostate cancer patients with biochemical recurrence of disease after undergoing definitive therapy, especially when serum PSA is >1.0 ng/ mL. In selected groups of patients with a high likelihood of regional or bone metastasis, pretreatment choline PET/CT is useful as an accurate and non-invasive staging tool. Nevertheless, it is important to be aware of the advantages and disadvantages of choline PET/CT and MRI for imaging in cases of prostate cancer.
References
- Kitajima K, Murphy RC, Nathan MA, Sugimura K. Update on positron emission tomography for imaging of prostate cancer. Int J Urol. 2014; 21: 12–23.
- Kitajima K, Yamamoto S, Fukushima K, Minamimoto R, Kamai T, Jadvar H. Update on advances in molecular PET in urological oncology. Jpn J Radiol. 2016; 34: 470–485.
- Kitajima K, Murphy RC, Nathan MA. Choline PET/CT for imaging prostate cancer: an update. Ann Nucl Med. 2013; 27: 581–591.
- Ackerstaff E, Glunde K, Bhujwalla ZM. Choline phospholopid metabolism: a target in cancer cells? J Cell Biochem. 2003; 90: 525–533.
- Hara T, Kosaka N, Kishi H. PET imaging of prostate cancer using carbon-11-choline. J Nucl Med. 1998; 39: 990–995.
- Hara T, Kosaka N, Kishi H. Development of 18F-fluoroethylcholine for cancer imaging with PET: synthesis, biochemistry, and prostate cancer imaging. J Nucl Med. 2002; 43: 187–199.
- Martorana G, Sciavina R, Corti B, Farsad M, Salizzoni E, Brunocilla E, et al. 11C-choline positron emission tomography/computerized tomography for tumor localization of primary prostate cancer in comparison with 12-core biopsy. J Urol. 2006; 176: 954–960.
- Soret M, Bacharach SL, Buvat I. Partial-volume effect in PET tumor imaging. J Nucl Med. 2007; 48: 932–945.
- Piert M, Park H, Khan A, Siddiqui J, Hussain H, Chenevert T, et al. Detection of aggressive primary prostate cancer with 11C-choline PET/CT using multimodality fusion techniques. J Nucl Med. 2009; 50: 1585–1593.
- Reske SN, Blumstein NM, Neumaier B, Gottfried HW, Finsterbusch F, Kocot D, et al. Imaging prostate cancer with 11C-choline PET/CT. J Nucl Med. 2006; 47: 1249–1254.
- Beheshti M, Imamovic L, Broinger G, Vali R, Waldenberger P, Stoiber F, et al. 18F Choline PET/CT in the preoperative staging of prostate cancer in patients with intermediate or high risk of extracapsular disease: a prospective study of 130 patients. Radiology. 2010; 254: 925–933.
- Poulsen MH, Bouchelouche K, Hoilund-Carlsen PF, Petersen H, Gerke O, Steffansen SI, et al. 18F-Fluorocholine positron-emission/computed tomography for lymph node staging of prostate cancer: a prospective study of 210 patients. BJU Int. 2012; 110: 1666–1671.
- Schiavina R, Scattoni V, Castelluci P, Picchio M, Corti B, Briganti A, et al. 11C-choline positron emission tomography/computed tomography for preoperative lymph-node staging in intermediate-risk and high-risk prostate cancer: comparison withclinical staging nomograms. Eur Urol. 2008; 54: 392–401.
- Contractor K, Challapali A, Barwick T, Winkler M, Hellawell G, Hazell S, et al. Use of [11C] choline PET-CT as a noninvasive method for detecting pelvic lymph node status from prostate cancer and relationship with choline kinase expression. Clin Cancer Res. 2011; 17: 7673–7683.
- Picchio M, Spinapolice EG, Fallanca F, Crivellaro C, GiovacchiniG, Gianolli L, et al. [11C] Choline PET/CT detection of bone metastases in patients with PSA progression after primary treatment for prostate cancer: comparison with bone scintigraphy. Eur J Nucl Med Mol Imaging. 2012; 39: 13–26.
- Fuccio C, Castellucci P, Schiavina R, Santi I, Allegri V, Pettinato V, et al. Role of 11C-choline PET/CT in the restaging of prostate cancer patients showing a single lesion on bone scintigraphy. Ann Nucl Med. 2010; 24: 485–492.
- Beheshti M, Vali R, Waldenberger P, Fitz F, Nader M, Hammer J, et al. The use of F-18 choline PET in the assessment of bone metastases in prostate cancer: correlation with morphological changes on CT. Mol Imaging Biol. 2009; 11: 446–454.
- Giovacchini G, Picchio M, Coradeschi E, Bettinardi V, Gianolli L, Scattoni V, et al. Predictive factors of [11C] choline PET/CT in patients with biochemical failure after radical prostatectomy. Eur J Nucl Med Mol Imaging. 2010; 37: 301–309.
- Cimitan M, Evangelista L, Hodolic M, Mariani G, Baseric T, Bodanza V, et al. Gleason score at diagnosis predicts the rate of detection of 18F-choline PET/CT performed when biochemical evidence indicates recurrence of prostate cancer: experience with 1,000 patients. J Nucl Med. 2015; 56: 209–215.
- Evangelista L, Zattoni F, Guttilla A, Saladini G, Zattoni F, Colletti PM, et al. Choline PET or PET/CT and biochemical relapse of prostate cancer: a systematic review and meta-analysis. Clin Nucl Med. 2013; 38: 305–314.
- Panebianco V, Sciarra A, Lisi D, Galati F, Buonocore V, Catalano C, et al. Prostate cancer: 1HMRS-DCEMR at 3T versus [18F] choline PET/CT in the detection of local prostate cancer recurrence in men with biochemical progression after Radical Retropubic Prostatectomy (RRP). Eur J Radiol. 2012; 81: 700–708.
- Kitajima K, Murphy RC, Nathan MA, Froemming AT, Hagen CE, Takahashi N, et al. Detection of recurrent prostate cancer after radical prostatectomy: comparison of 11C-choline PET/CT with pelvic multiparametric MR imaging with endorectal coil. JNucl Med. 2014; 55: 223–232.
- Souvatzoglou M, Eiber M, Martinez-Moeller A, Furst S, Holzapfel K, Maurer T, et al. PET/MR in prostate cancer: technical aspects and potential diagnostic value. Eur J Nucl Med Mol Imaging. 2013; 40: 79–88.
- Wetter A, Lipponer C, Nensa F, Heusch P, Rubben H, Altenbernd JC, et al. Evaluation of the PET component of simultaneous [18F]choline PET/MRI in prostate cancer: comparison with [18F]choline PET/CT. Eur J Nucl Med Mol Imaging. 2014; 41: 79–88.
- Freitag MT, Radtke JP, Hadaschik BA, Kopp-Schneider A, Eder M, Kopka K, et al. Comparison of hybrid 68Ga-PSMA PET/MRI and 68Ga-PSMA PET/CT in the evaluation of lymph node and bone metastases of prostate cancer. Eur J Nucl Med Mol Imaging. 2016; 43: 70–83.