Editorial
Extracellular Matrix in the Tumor Stroma as a Therapeutic Tool: The Good or The Bad?
Nicoletta Gagliano*
Department of Biomedical Sciences for Health, University of Milan, Italy
*Corresponding author: Nicoletta Gagliano, Department of Biomedical Sciences for Health, University of Milan, Fax: 39 02 50315387; Tel: 39 02 50315374, Italy
Published: 31 Jan, 2017
Cite this article as: Gagliano N. Extracellular Matrix in the
Tumor Stroma as a Therapeutic Tool:
The Good or The Bad?. Clin Oncol.
2017; 2: 1197.
Editorial
Pancreatic Ductal Adenocarcinoma (PDAC) is the fourth leading cause of cancer mortality in
the United States, with overall 5-year survival of less than 7%, due to the high incidence of recurrence
and metastases dissemination [1,2].
During PDAC progression, the stroma of the pancreas undergoes evident qualitative and
quantitative modifications. In fact, PDAC is characterized by an intense “desmoplastic reaction”,
defined as the host fibrotic response to the invasive carcinoma, consisting in the abnormal
accumulation of stromal components, mostly collagen fibers.
The stroma in the tumor microenvironment contains Extracellular Matrix (ECM) components,
growth factors and soluble mediators, and different stromal cells including fibroblasts, inflammatory
and pancreatic stellate cells, influencing cancer cell phenotype, behavior and chemoresistance [3-5].
The ECM is particularly important in PDAC since the desmoplastic reaction represents the
histological hallmark of PDAC, often accounting for 50-80% of the tumor volume [3,4].
The stroma in the microenvironment is where cancer cells are embedded, and stromal ECM
components act as a physical scaffold, facilitating interactions between different cell types, provide
survival and differentiation signals andaffect resistance to anticancer drugs. ECM has been
determined to be an important mediator of cancer cell behavior, influencing tumor cell proliferation
and migration [6] and tissue homeostasis. The ECM also influences cell polarity and angiogenesis
[7].
Key ECM components in the desmoplastic reaction have been identified, such as collagen type
I (COL-I), IV (COL-IV) and V (COL-V), fibronectin, laminin [8]. COL-I is the most abundant
and was associated with increased integrin mediated cell-cell adhesion, proliferation and migration
of PDAC cells [9]. In addition, the oncofetal type I-trimer collagen formed by homologous alpha
1 chains and lacking regular alpha 2(I) chains was detected [10] and reported as an inducer of
active proliferation and motility in breast cancer cells. Its expression was suggested to facilitate
cell migration and invasion [11]. Basement membrane components such as COL-IV and laminin
provide a proper microenvironment for PDAC cells decreasing the cytotoxicity of anti-cancer drugs,
and inducing cancer cell growth [6]. The role of COL-V, a minor component of ECM, remains
poorly understood since it triggers opposite cellular responses depending on the cell type. In breast
cancer, type V collagen impairs breast ductal infiltrating carcinoma cells survival by promoting
apoptosis [12], and its decrease was associated to increased tumor growth rate, motility and invasion
in lung cancer, as well as to increased angiogenesis [13]. Its role in PDAC is not described yet.
Fibronectin is a key ECM component, influencing collagen type I deposition in fibrotic processes
[14]. The evidence of large quantities of fibronectin in both chronic pancreatitis and pancreatic
cancer suggests that this protein may facilitate the development of PDAC [15]. Hyaluronan (HA)
was shown to be involved in the invasion of PDAC cells and a more than 4-fold increase of HA at the
invasive tumor front, relative to the adjacent normal tissue, was reported [16]. High levels of COL-I,
COL-IV or HA significantly reduced overall survival of PDAC patients [17].
Desmoplasia characterizes PDAC and, interestingly, it was demonstrated that primary tumors
and metastatic lesions exhibit by similar levels of desmoplasia, including high levels of some ECM
components such as COL-I, COL-III and COL-IV [17]. The expression of markers of desmoplasia
was analyzed and it was demonstrated that desmoplasia is also detected in metastatic sites [18].
Therefore, metastatic lesions are also fibrotic as primary tumors are. This furtherly confirms the key
role played by ECM components in the desmoplastic reaction of PDAC.
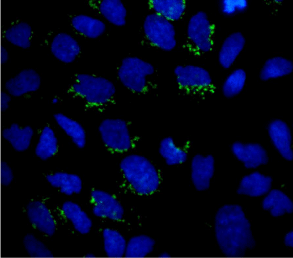
A key player in the development and maintenance of desmoplasia
is the Pancreatic Stellate Cell (PSC), involved in the secretion of ECM
components in the fibrotic tissue, but also PDAC cells secrete ECM
components such as COL-I (Figure 1) (Gagliano, unpublished data).
Considered the role of desmoplasia in PDAC and since a negative
correlation between ECM components, such as collagen, and the
delivery of macromolecules and possible therapeutic compounds
exists, it was hypothesized that targeting the fibrotic stroma of PDAC
could represent a benefit also for PDAC therapy and, therefore, an
appealing therapeutic target.
However, stromal depletion, either by conditional deletion or
targeting of the sonic hedgehog pathway [19] or by depletion of
activated my fibroblasts [20], resulted in more aggressive tumors.
In fact, it was recently demonstrated [19] that some components of
the stroma have a tumor-promoting role, while other components
could be tumor-suppressive, and the final effect is dependent on
the differentiation grade of cancer cells. These findings suggested
that the complete destruction of some components in the tumor
microenvironment can potentially promote tumor growth. Therefore,
the influence of desmoplastic components on PDAC cells could
be context dependent and the bidirectional and mutual cross-talk
between stroma and PDAC cells should be analyzed.
If stromal ablation seems not effective, recent studies point
to stromal “normalization” as a new therapeutic approach for
the treatment of PDAC to restore the homeostasis in the tumor
microenvironment [21]. Accordingly, in a genetically engineered
mouse model of pancreatic cancer it was demonstrated that the
reprogramming of the tumor stroma, by rendering activated PSCs
physiologically quiescent, results in tumor regression and increases
drug delivery, resulting in asignificant increase in median survival.
The combination of the restoration of the homeostasis in the
desmoplastic stroma with an anti-tumor cytotoxic therapy targeting
cancer cells could represent a new goal for a more effective therapeutic
approach in PDAC. In fact, a reduction in cancer cell proliferation
and invasion, and enhanced cell apoptosis were demonstrated after
treatment of PDAC organotypic cultures with a combination of two
different drugs, all-trans retinoic acid (ATRA) and gemcitabine [22].
In this study, it was demonstrated that PSC activity (measured by
deposition of ECM proteins such as collagen type I) and PSC invasive
potential were both reduced after combination therapy. These recent
findings suggest to target both PDAC cancer cells and the stroma, in
order to exert a therapeutic control of PDAC progression.
These data reinforce the importance of fully understanding the
intricate cellular interactions with ECM components in the tumor
microenvironment and suggest that the role of ECM in PDAC
progression must be furtherly explored, in order to create sufficient
biological insight in cell-ECM cross-talk. This will lead to find more
effective therapeutic tools able to restore tumor microenvironment
homeostasis and, at the same time, to revert the malignant phenotype
to normal cell phenotype of PDAC cells.
Figure 1
Figure 1
Micrograph showing COL-I expression (green) in some scattered
PDAC cells grown on round coverslips. Nuclei are stained with DAPI. Original
magnification: 60x.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, CA Cancer J Clin. 2015; 65: 5-29.
- Ghaneh P, Costello E, Neoptolemos JP. Biology and management of pancreatic cancer. Gut. 2007; 56: 1134-1152.
- Chu GC, Kimmelman AC, Hezel AF, DePinho RA. Stromal biology of pancreatic cancer. J Cell Biochem. 2007; 101: 887-907.
- Erkan M, Hausmann S, Michalski CW, Fingerle AA, Dobritz M, Kleeff J, et al. The role of stroma in pancreatic cancer: diagnostic and therapeutic implications. Nat Rev GastroenterolHepatol. 2012; 9: 454-467.
- Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001; 1: 46-54.
- Miyamoto H, Murakami T, Tsuchida K, Sugino H, Miyake H, Tashiro S. Tumor-stroma interaction of human pancreatic cancer: acquired resistance to anticancer drugs and proliferation regulation is dependent on extracellular matrix proteins. Pancreas. 2004; 28: 38-44.
- Park CC, Bissell MJ, Barcellos-Hoff MH. The influence of the microenvironment on the malignant phenotype. Mol Med Today. 2000; 6: 324-329.
- Mahadevan D, Von Hoff DD. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2007; 6: 1186-1197.
- Grzesiak JJ, Bouvet M. The alpha2beta1 integrin mediates the malignant phenotype on type I collagen in pancreatic cancer cell lines. Br J Cancer. 2006; 94: 1311-1319.
- Pucci-Minafra I, Luparello C, Andriolo M, Basiricò L, Aquino A, Minafra S. A new form of tumor and fetal collagen that binds laminin. Biochemistry. 1993; 32: 7421-7427.
- Pucci-Minafra I, Albanese NN, Di Cara G, Minafra L, Marabeti MR, Cancemi P. Breast cancer cells exhibit selective modulation induced by different collagen substrates. Connect Tissue Res. 2008; 49: 252-256.
- Luparello C, Sirchia R. Type V collagen regulates the expression of apoptotic and stress response genes by breast cancer cells. J Cell Physiol. 2005; 202: 411-421.
- Souza P, Rizzardi F, Noleto G, Atanazio M, Bianchi O, Parra ER, et al. Refractory remodeling of the microenvironment by abnormal type V collagen, apoptosis, and immune response in non-small cell lung cancer. Hum Pathol. 2010; 41: 239-248.
- Ignotz RA, Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986; 261: 4337-4345.
- Binkley CE, Zhang L, Greenson JK, Giordano TJ, Kuick R, Misek D, et al. The molecular basis of pancreatic fibrosis: common stromal gene expression in chronic pancreatitis and pancreatic adenocarcinoma. Pancreas. 2004; 29: 254-263.
- Bertrand P, Girard N, Delpech B, Duval C, d'Anjou J, Dauce JP. Hyaluronan (hyaluronic acid) and hyaluronectin in the extracellular matrix of human breast carcinomas: comparison between invasive and non-invasive areas. Int J Cancer. 1992; 52: 1-6.
- Whatcott CJ, Diep CH, Jiang P, Watanabe A, LoBello J, Sima C, et al. Desmoplasia in Primary Tumors and Metastatic Lesions of Pancreatic Cancer. Clin Cancer Res. 2015; 21: 3561-3568.
- Duda DG, Duyverman AM, Kohno M, Snuderl M, Steller EJ, Fukumura D, et al. Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci U S A. 2010; 107: 21677-21682.
- Rhim AD, Oberstein PE, Thomas DH. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014; 25: 735-747.
- Özdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, et al. Depletion of carcinoma-associatedfibroblasts and fibrosisinducesimmunosuppression and accelerates pancreas cancer with reducedsurvival. Cancer Cell. 2014; 25: 719-734.
- Froeling FE, Kocher HM. Homeostatic restoration of desmoplastic stroma rather than its ablation slows pancreatic cancer progression. Gastroenterology. 2015; 14: 849-850.
- Carapuça EF, Gemenetzidis E, Feig C, Bapiro TE, Williams MD, Wilson AS, et al. Anti-stromal treatment together with chemotherapy targets multiple signalling pathways in pancreatic adenocarcinoma. J Pathol. 2016; 239: 286-296.