Review Article
Role of Radium-223 in the Treatment of Metastatic Castration Resistant Prostate Cancer (mCRPC): Clinical Practice and Future Perspectives
Emilio Bombardieri1*, Giovanni Luca Ceresoli2, Lucia Setti1, Maria Bonomi2, Elisa Villa3,
Gianluigi Ciocia1, Riccardo Vicinelli4 and Laura Evangelista5
1Department of Nuclear Medicine, Humanitas Gavazzeni, Bergamo, Italy
2Department of Clinical Oncology, Humanitas Gavazzeni, Bergamo, Italy
3Department of Radiotherapy, Humanitas Gavazzeni, Bergamo, Italy
4Department of Nuclear Medicine, University Milano Bicocca, Italy
5Department of Radiotherapy and Nuclear Medicine, 10V, Padova, Italy
*Corresponding author: Emilio Bombardieri, Department of Nuclear Medicine, Humanitas Gavazzeni, Via Mauro Gavazzeni 21, 24125 Bergamo, Italy
Published: 30 Dec, 2016
Cite this article as: Bombardieri E, Ceresoli GL, Setti L,
Bonomi M, Villa E, Ciocia G, et al.
Role of Radium-223 in the Treatment
of Metastatic Castration Resistant
Prostate Cancer (mCRPC): Clinical
Practice and Future Perspectives. Clin
Oncol. 2016; 1: 1173.
Abstract
The therapeutic landscape for patients with metastatic castration Resistant prostate cancer (mCRPC)
has rapidly changed in the last 5 years. New hormonal, cytotoxic and immunological agents have
been introduced demonstrating efficacy both in terms of cancer control and survival improvement.
223Ra-dichloride (Radium-223) is a calcium-mimetic alpha emitting radiopharmaceutical agent with
a very high linear energy transfer, label to determine an irreversible damage in the DNA of cancer
cells. In a large controlled randomized perspective clinical trial, Radium-223 provided interesting
results in symptomatic mCRPC patients with bone metastases, by decreasing pain, delaying Skeletal
Related Events (SREs) and improving the survival. Among a series of radiopharmaceuticals for
the treatments of skeletal metastases (i.e., Strontium-89, Rhenium-186 and Samarium-153) in
prostate cancer patients, Radium-223 is the first agent that demonstrated a favorable impact on
both improvement of quality of life and overall survival.
This overview discusses the current armamentarium available for mCRPC patients, focusing the
attention on Radium-223, its selective uptake in bone metastases, the safety profile and the open
questions related to its use in clinical practice, such as the doses and the number of cycles of
treatment. Moreover, being the mechanism of Radium-223 action not potentially in overlap with
any other available treatments, it results suitable for both sequencing and combination studies. In
the present paper, future perspectives are briefly discussed by the authors considering some possible
associations of Radium-223 with other therapeutic agents that would improve the outcomes of
patients without increasing toxicities, and by looking for its potential applications in the next future.
Keywords: Castration Resistant Prostate Cancer; Bone metastases; Radium-223, Radio metabolic therapy
Introduction
Prostate Cancer (PCa) is the second most commonly diagnosed cancer in men and accounts
for nearly 20% of all newly diagnosed male tumors. At diagnosis, approximately 80% of patients
present with localized PCa and 4% with distant metastases: the 5-year relative survival rate is 100%
and 28% respectively [1]. Due to cell growth dependence on androgens, Androgen Deprivation
Therapy (ADT) is the standard of care for advanced or metastatic PCa patients. However, following
initial response to ADT, approximately 10% to 20% of patients (and virtually all patients with
metastatic disease) will develop Castration-Resistant Disease (CRPC), an incurable condition with
a median survival of < 3 years [2]. More than 90% of men with Metastatic Castration-Resistant
Prostate Cancer (mCRPC) have radiological evidence of bone metastases [3], often leading to
symptomatic skeletal events with pain and bone fractures [3-6]. Anemia accompanies advancing
disease and is a risk factor for poor outcome in mCRPC [7,8]. Since 2004, docetaxel in combination
with prednisone has been the standard therapy for patients with mCRPC [9-12]. However, in the
last 5 years, several new agents with different mechanism of action have become available for the
management of these patients. These compounds include a new taxane, cabazitaxel, the second-generation hormonal agents abiraterone and enzalutamide, and the alpha emitter Radium-223 [13-20].
Improving options for patients with mCRPC requires a different
approach to each patient, to offer the most appropriate therapy. A
multidisciplinary team should follow the patient with prostate cancer
since diagnosis, to integrate the different professional knowledge and
skills and to plan an optimal patient treatment [21]. In the last years,
there has been increasing interest for radiopharmaceutical agents able
to specifically target the bone or the cancer [22]. Several treatment
modalities are used to control metastatic bone pain or prevent
Skeletal-Related Events (SREs) from PCa, such as radiotherapy,
186Rh and 153Sm-ethylene diamine tetra methylene phosphonate,
bisphosphonates, denosumab and other bone-seeking agents;
however, only Radium-223 has demonstrated to improve survival,
showing not only a bone targeted action, but also an anti neoplastic
effect [20,23-27].
Radium-223 dichloride (Radium-223) is a calcium mimetic
that specifically targets newly formed bone in areas of osteoblastic
metastases. It decays by emitting high-energy alpha particles causing
predominantly on-repairable double-stranded DNA breaks in tumor
cells [28-30]. Tissue penetration is minimal, resulting in highly
localized cell killing with negligible damage to surrounding healthy
tissues including bone marrow cells [28,29]. Unlike External Beam
Radiation Therapy (EBRT) and beta particle emitting radionuclides
indicated for pain palliation, the short range of therapeutic
Radium-223 alpha particles spares hematologic tissue, which may
result in fewer hematologic Adverse Events (AEs) [31].
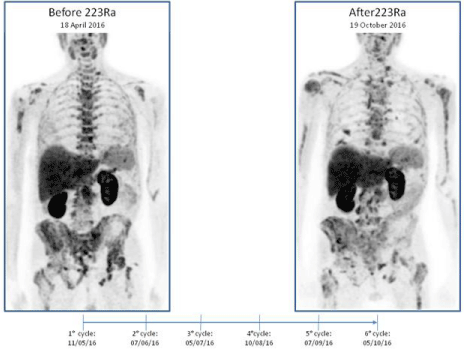
Figure 1
Figure 1
18F-Choline PET/CT scan in a 69-year-old patient before (A) and after 6 cycles (B) of 223Ra-dicholoride therapy.
Role of the Multidisciplinary Team
Since 2010, the approval of cabazitaxel, oral agents abiraterone
acetate and enzalutamide and Radium-223 has expanded dramatically
the treatment options for mCRPC patients, resulting in longer
survival and improved quality of life [8,15-18,20].
Patient selection, the opportunity to combine treatments with
other modalities, and the optimal treatment sequencing are still
matter of debate. Furthermore, counseling on patient expectations in
terms of prognosis and quality of life is becoming more and more
important in formulating the best individual treatment plan. For these
reasons, a multidisciplinary approach should become the standard of
care for the treatment of patients with mCRPC; specialized urologists,
medical oncologists, radiation oncologists, nuclear medicine
physicians, pathologists, imaging specialists, psychologists, experts in
rehabilitation, experts in supportive and palliative care, geriatricians
[32,33] should work together in a structured patient-focused
multidisciplinary setting. These figures have a specific role depending
on the patient’s disease state and, to be effective and efficient, the
various members should be organized taking into consideration the
different phases of the disease and associated treatments. Synchronous
counseling avoids separate meetings and reduces patient anxiety.
Therapeutic Landscape in mCRPC
Chemotherapy
In 2004, two pivotal trials have demonstrated the possibility for
chemotherapy to achieve not only disease palliation (until that date,
the only documented efficacy for mitoxantrone chemotherapy was
palliation), but also to have a statistically significant effect on patient
survival. The SWOG 99-16 trial randomized patients to receive either
mitoxantrone and prednisone, or the combination of docetaxel
plus estramustine phosphate [14]. The TAX 327 trial compared the
combination of mitoxantrone and prednisone versus docetaxel and
prednisone [13]. In this study docetaxel was given at two different
schedules, i.e., either weekly or 3-weekly. Both studies showed that
docetaxel combinations were significantly superior to mitoxantrone
and prednisone in Overall Survival (OS). It is difficult to compare
these two landmark trials. However, the addition of estramustine
seems to add no benefit, while increasing toxicity [14]; therefore,
the 3-weekly docetaxel schedule was approved as the recommended
front-line regimen for mCRPC. Subsequent analysis of the TAX 327
trial has demonstrated the superiority of docetaxel plus prednisone
combination in all patient subgroups, irrespectively of age, tumor
burden, site of metastasis, bone involvement and the presence of
disease related symptoms [34].
In more recent years, cabazitaxel, a semi synthetic analog of
docetaxel, has been approved for mCRPC patients progressing during
or after docetaxel treatment. The TROPIC trial was a randomized trial
comparing mitoxantrone versus cabazitaxel, both in combination
with low dose prednisone, in 755 patients after first line treatment
with docetaxel and prednisone. This study showed a statistically
significant OS advantage in favor of cabazitaxel (15.1 vs. 12.7 months,
p <0.0001). Cabazitaxel documented also significant better outcomes
for PFS, objective response of measurable disease and PSA response.
However, pain control was comparable in the two arms and a higher
number of adverse events occurred in the cabazitaxel arm [15].
Hematological toxicities were more frequent and severe; therefore a
careful patient selection and appropriate prophylactic use of G-CSF
should be considered when using this agent [35].
Second generation hormonal therapies
Until recently, options for the management of CRPC patients have
been limited to second-line attempts with agents like corticosteroids,
high dose estrogens, ketoconazole. The use of these compounds, which
have been shown to benefit no more than 30% of patients without any
clear advantage in survival, was made on empirical bases [36]. Over
the past few years, the driving role of AR has been evidenced even in
the castration resistant disease setting, because of genetic alterations,
either amplification or mutations, of the Androgen Receptor (AR),
which can allow tumor growth still driven by the binding of amplified/
mutated AR with residual androgens. In this context, new endocrine
therapies have been developed.
Abiraterone acetate is a potent, selective and irreversible inhibitor
of 17-alpha-hydroxylase and C-17, 20-lyase CYP17 activity, thereby
blocking non-gonadal production of androgens. It is associated with
low dose corticosteroids to minimize the incidence and relevance of
side effects like hypertension and hypokalemia. The COU-AA 301
trial compared abiraterone plus prednisone versus prednisone plus
placebo in patients with docetaxel-pretreated mCRPC; this trial
showed a definite superiority of abiraterone over placebo both in
terms of OS (with a median of 14.8 months in the abiraterone group
vs. 10.9 months in the placebo group; p<0.00001) and in terms of
radiologic progression (5.6 months vs. 3.6 months, respectively),
PSA response (29% vs. 6%) and pain control (44% vs. 27%) [16].
Abiraterone acetate was more effective in decreasing the incidence
and severity of SREs. Side effects were generally mild, with a low rate
of discontinuations. Based on these results, the COU-AA 302 trial
was conceived to investigate the role of the drug in docetaxel-naïve
patients with mCRPC. The selected patients had asymptomatic or
mildly symptomatic disease and no evidence of visceral metastases.
Abiraterone was statistically superior to prednisone, either in
terms of OS (34.7 months vs. 30.3 months; p<0.003) and in terms
of radiological PFS (16.5 months vs. 8.3 months; p<0.001). The
incidence of side effects in the two treatment groups was comparable
to that observed in COU-AA 301 study, with a significant increase in
cardiac disorders (19% vs. 16%) and altered liver function tests (12%
vs. 5%) in the abiraterone arm [16,17].
Enzalutamide is a new potent anti-androgen, with no agonistic
activity and a greater affinity for the AR than first generation antiandrogens
like fltamide or bicalutamide. It also acts on nuclear
translocation and DNA binding of AR. The AFFIRM trial enrolled
1199 patients affected by mCRPC progressed under or after a
treatment with docetaxel [18]. Patients were randomized to receive
enzalutamide or placebo. At a median follow-up time of 14.4 months,
median OS was 18.4 months in the enzalutamide group vs. 13.6 months
in the placebo group (HR: 0.63; 95% CI: 0.52-0.75; p<0.0001). Time to
radiological progression (8.3 months vs. 2.9 months) and time to PSA
progression (8.3 months vs. 3 months) also statistically favored the
enzalutamide arm. A significant impact on the incidence and severity
on SREs was observed with enzalutamide. More patients assigned to
enzalutamide experienced fatigue, diarrhea, muscle-skeletal troubles
and hot flushes, and five patients assigned to enzalutamide developed
seizures [18].
Enzalutamide was evaluated in chemo-naïve patients in
the PREVAIL trial; 1680 patients with asymptomatic or mildly
symptomatic mCRPC were randomized to receive either enzalutamide
or placebo. A significant increase in OS and radiological PFS was
documented. An advantage in favor of enzalutamide was also shown in respect to time to chemotherapy initiation (HR 0.35), time to first SRE (HR 0.72) and time to PSA progression (HR 0.17) [19].
Zoledronic acid and denosumab
Zoledronic acid is the only bisphosphonate that has demonstrated
significant efficacy and long-term clinical benefit by preventing SREs
in patients with PCa. The administration of zoledronic acid (4 mg
every 3 weeks) versus placebo in 643 mCRPC patients resulted in
a reduction of the number of patients having a SRE (33% vs. 44%;
P=0.021). It also showed improvements in pain and analgesia scores
but there were no differences in disease progression or OS [37-40].
Denosumab is a fully human monoclonal antibody directed
against RANKL, the main driver of osteoclast formation, function,
and survival. It acts inhibiting osteoclast-mediated bone destruction,
decreasing bone re-absorption and increasing bone mass. The drug
is administered via subcutaneous injection. A phase III trial on
1904 mCRPC patients compared denosumab (120 mg administered
subcutaneously every 4 weeks) with zoledronic acid (4 mg
intravenously every 3 weeks) [41,42]. Denosumab prolonged the time
to first SRE by 3.6 months (20.7 months vs. 17. 1 months; HR=0.82;
P <0.001 for non-inferiority, P=0.008 for superiority). The two
groups had similar OS and time to disease progression. OS, disease
progression, and rates of AEs and serious AEs were similar in the two
arms, but denosumab had an increased incidence of hypocalcemia
(13% vs. 6% in the zoledronic acid group; P<0.0001).
Beta emitters
Until a few years ago, nuclear medicine proposed some betaemitting
agents for the treatment of bone metastases, such as
Strontium-82, Samarium-153 and Rhenium-186, that demonstrated
only a palliative action in patients with diffuse skeletal disease [22].
Rhenium-186-HEDP Imaging of the 155 keV gamma photon is
an advantage which provides an opportunity for estimation of
radiation dose to metastatic sites. Beta Emitters Strontium-89 and
Samarium-153 documented an advantage in pain palliation and no
benefit on survival. As β-emitters have a track length in the order of
millimeters, their use for the palliation of bone pain from metastases
has been limited by bone marrow toxicity [43]. A systematic review
and meta-analysis of clinical studies involving Strontium-89 and
Samarium-153 showed overall efficacy of 70% for both agents in
reducing metastatic bone pain in mCRPC patients and complete pain
relief in 27% of patients (REF). Dose-response studies have shown
increasing rates of response to pain and increasing myelotoxicity with
increasing doses of Samarium-153, limiting the use of higher doses
[44,45].
A phase 3, placebo-controlled Canadian clinical trial evaluating
the efficacy of a single 10.8 mCi injection of strontium-89 as an
adjuvant to local-field radiotherapy in mCRPC patients(n = 126)
documented significant delay in pain progression, producing as
expected higher hematologic toxicity involving leukocytes and
platelets. Complete pain response was observed in 30–60% of treated
patients, with no statistically significant difference in survival [46,47].
Samarium-153 showed pain palliation in 152 patients with mCRPC
and painful bone metastases in a pivotal phase 3 trial [48]. A statistically
significant reduction in opioid use, suggesting pain reduction, was
observed at treatment weeks 3 and4. In the TRAPEZE phase 2/3
trial in 757mCRPC patients, strontium-89 treatment after 6 cycles
of docetaxel improved clinical progression-free survival (HR 0.85; 95
% CI 0.72–0.99; p = 0.036) [49]. A phase 2 trial of a consolidation
regimen of samarium-153-EDTMP with docetaxel in mCRPC after
docetaxel and hormonal therapy, showed that the combination was
well tolerated and produced sustained pain relief and a PSA response
[50,51]. Samarium-153 was safely used in prostate cancer patients
who had prior chemotherapy or radiotherapy.
Radium-223
Radium-223 (223Ra), a first-in-class alpha-emitting
radiopharmaceutical, is an alkaline earth element and acts like a
calcium mimetic: it is absorbed into bone matrix at sites of osteoblastic
activity. The half-life of 223Ra is t1/2 =11.4 days, leading to interest in
its use in cancer treatment as the drug can be delivered to the site of
bone disease and continue to deliver dose. Radium-223 decays to the
stable isotope of lead, 207Pb, in six steps. Of the energy emitted, 95.3%
decays as alpha radiation with 3.6% and 1.1% as beta and gamma
radiation, respectively. It is possible to detect photon emissions from
the decay of Radium-223 using standard techniques. Alpha particles
penetrate tissue only to a depth of 2-10 cell diameters (<100 micron),
leading to highly localized cell killing and minimal damage to normal
tissues. The favorable path length of the emitted radiation warrants
therefore bone marrow sparing.
After intravenous injection, 223Ra is cleared rapidly from the
blood; only 6% of initial activity is seen in the blood by 1-hour postinjection,
less than 1% at 24hours. Excretion is predominantly via the
gastrointestinal tract with minimal (approximately 5%) early urinary
excretion [52,53]. Evidence that 223Ra accumulates in the bones has
been demonstrated by scintigraphy in phase I trial. Low external
dose rates [54] allow for patient release from radiation control
measures immediately following administration. Minimal prudential
restrictions on family contacts are therefore needed after treatment
with 223Ra. As there is also some blood and urine activity, caution
is recommended with body fluids and stool for one week after drug
injection.
223Ra has documented a safe profile in a phase I trial, with no
observed DLT [28-30]; the MTD was not reached in CRPC patients
and metastatic breast cancer patients treated at different dosing
schedules. Phase I and phase II trials have shown the safety of the
drug and the effectiveness on Alkaline Phosphatase (ALP) reduction
and pain reduction. The pivotal trial ALSYMPCA demonstrated
an OS benefit of 223Ra as compared to placebo administration for
patients with symptomatic bone metastases from mCRPC. Enrolled
patients had at least two bone metastases at bone scan and no visceral
disease on CT scan; lymph node disease up to 3 cm of diameter in
short axis was allowed. They should have received docetaxel, or be
unfit for chemotherapy or have refused it. Symptomatic disease was
defined including patients with regular assumption of analgesic
medication (non-opioid or opioid) or pain-free patients who had
received EBRT for cancer-related bone pain in the 12 weeks before
randomization. At baseline, 44% of 223Ra and 45% of placebo patients
had no pain or had mild pain effectively managed without need for
opioids. Patients in the non-opioid subgroup presented less advanced
disease: a greater proportion with ALP values <220 U/l, lower median
ALP and lactate dehydrogenase values, better performance status, less
extensive skeletal disease, fewer prior docetaxel therapy and EBRT
for pain. Enrolled patients were stratified in accord with previous
chemotherapy (yes vs. not), concomitant bisphosphonate use (yes
vs. not) and ALP baseline level. In this randomized phase 3 study,
223Ra plus best standard of care (BSoC) versus placebo plus BSoC
prolonged median OS by 3.6 months (Hazard Ratio [HR]=0.70; 95% confidence interval [CI]: 0.58–0.83; p<0.001; median 14.9 months vs.
11.3 months, respectively): The effectiveness of 223Ra was documented
in all the stratified subgroups. 223Ra also prolonged median time to
first Symptomatic Skeletal Event (SSE) by 5.8 months (HR=0.66;
95% CI: 0.52–0.83; p<0.001; median 15.6 months vs. 9.8 months,
respectively) [20,55]. These results led to 223Ra approval for the
treatment of mCRPC patients with symptomatic bone metastases and
no known visceral metastatic disease.
Patients receiving 223Ra had a meaningful improvement in
quality of life as defined by an increase of ≥ 10 points on a scale of
0 to 156 on the FACT-P questionnaire (25% vs. 16% for 223Ra and
placebo respectively; P=0.02). The survival duration and time to first
SSE were longer in minimally symptomatic (i.e., WHO ladder pain
score 0–1/without opioid use) than in more symptomatic patients
(i.e., WHO ladder pain score 2–3 with opioid use). These data suggest
that appropriate timing of 223Ra treatment should not be based on
symptom severity and that using 223Ra earlier may optimize clinical
outcome and allow sequencing with other effective therapies. In
addition, 223Ra treatment significantly delayed time to first opioid use
and reduced the need of EBRT for bone pain, but ALSYMPCA was
not designed to evaluate the effect of 223Ra on pain, since the primary
endpoint was OS. Accordingly, any observed pain response or lack of
response should not be considered a cause to prematurely stop 223Ra
treatment.
Radium-223 had a favorable safety profile, with a low overall
incidence of grades 3-4 myelosuppression and fewer AE and SAE
than placebo arm [20]. The drug was well tolerated, regardless of
prior docetaxel exposure. No differences were seen in the safety
profile between patients who did and did not receive concomitant
EBRT for bone pain during the study.
The most important recorded toxicities were minor gastrointestinal
side effects and mild neutropenia and thrombocytopenia [20]. No
significant differences were reported between treatment arms in
anemia, as this event was mainly related to baseline extent of bone
disease. A number of ≥6 bone metastases was associated with increased
risk of grade 2 – 4 anemia (HR = 1.52; 95% CI: 1.17 – 1.97; p = 0.002).
The number of blood transfusions and time to first blood transfusion
were similar among the two groups [31]. Risk for developing G2-4
neutropenia was related to prior docetaxel therapy, higher WHO pain
score and decreased baseline neutrophil count; platelets decrease was
related to previous docetaxel administration and lower basal level of
platelets and hemoglobin: these features were generally associated to
a higher extent of disease, and to higher ALP level and bone pain.
Platelets transfusions were more frequently administered to 223Ra
treated patients, mainly after third cycle, suggesting a cumulative
effect and advising clinicians to carefully evaluate the benefit and risk
of continuing treatment.
It is important to identify potential risk factors for hematologic
toxicity before 223Ra initiation, to monitor high-risk patients for
treatment modifications [31]. The maximum efficacy of treatment
is associated to completion of 6 injections administration, and as
above mentioned the tolerability is better in presence of adequate
level of ALP, hemoglobin and platelets, and in patients with
lower extension of skeletal disease and mild pain [20,56]. Current
international guidelines recommend 223Ra in an option in both preand
post-docetaxel settings, and it is possible to administer 223Ra in
patients with bone metastases also as first line therapy for mCRPC
[57,58]. No safety concern was identified in an exploratory analysis
of prospectively collected data from ALSYMPCA trial on patients
that received subsequent chemotherapy after 223Ra or placebo [59].
Most analyzed patients had also received docetaxel prior to 223Ra. No
significant differences were underlined between treatment groups in
frequency of grade 3-4 hematological adverse events, indicating that
the use of chemotherapy following 223Ra is feasible regardless of prior
docetaxel use [59], and, most importantly, that prior treatment with
223Ra do not compromise the efficacy of subsequent chemotherapy.
This observation strengthens the possible use of 223Ra as first line
approach, when tumor burden is limited, hemoglobin level adequate
and patient is more likely to complete the planned treatment.
Overall, the treatment is safe also in a long-term period of
observation: at the end of the 3-year follow-up period, no reports
of acute myeloid leukemia, myelodysplastic syndrome or new
primary bone cancer are known [22]. A study exploring the benefit
of retreatment with 223Ra in 44 patients who have already received 6
cycles documented a lower number of hematological events than in
the ALSYMPCA trial. Besides prior 223Ra, all patients had received
previously ≥2 hormonal regimens; 45% had been pretreated with
≥1 chemotherapy regimen. Overall, their baseline characteristics
were comparable to ALSYMPCA. Twenty-nine (66%) completed
all the 6 retreatment injections. No new safety concerns were noted;
only 2 patients had grade 3 hematological adverse events. Only one
patient had radiographic bone progression, with a median rPFS of 9.9
months [59].
The international Expanded Access Programme (EAP) was a
phase 3b trial conducted after ALSYMPCA and before regulatory
approval; the endpoints were safety and OS. A total of 839 patients
with bone metastases (at least two lesions) from CRPC and without
visceral disease were enrolled. Lymph nodes were allowed up to
3 cm in diameter, and patients could be treated independently
if symptomatic or asymptomatic. Also concomitant anticancer
therapies were allowed. Overall, 696 patients received at least
one dose of 223Ra and were evaluated for safety. Grade 3-4 anemia
occurred in 5% of patients, thrombocytopenia in 2% and neutropenia
in 1%. Median OS was 16 months, and it was longer for patients with
normal ALP than for patients with higher ALP levels; for patients with
baseline hemoglobin level of 10g/dL or greater versus patients with
lower Hb; for patients with ECOG PS of 0 compared to ECOG PS 1;
and for patients with no reported baseline pain versus symptomatic
patients. Median OS was also better in patients receiving concomitant
denosumab and in patients with concomitant administration of 223Ra
and enzalutamide or abiraterone acetate [56].These observations
confirm the data on efficacy and safety reported in the registration
trial, and reinforce the magnitude of benefit in the early use of the
drug. Furthermore, preliminary evidence of feasibility and efficacy of
combination therapies with 223Ra and new-generation antiandrogens
was shown.
Disease evaluation with Radium - 223
More patients in the 223Ra group had a ≥30% reduction in the total
ALP and PSA than in the placebo arm. A significant prolongation
in time to increase in ALP was seen with 223Ra compared to placebo
(7.4 months vs. 3.8 months respectively; HR 0.17; 95% CI 0.13 to
0.22; P <0.001). There was no significant difference in time to PSA
progression. PSA test should not be used to measure response during
therapy with 223Ra, because its level typically continues to rise during
the early phase of treatment courses. A decline in PSA level is usually
observed in responding patients after 4 or 5 months of treatment, which is too late for assessing response. As evidenced in recent
case reports [61], we recommend not to discontinue 223Ra therapy
on the occurrence of an asymptomatic PSA rise not supported by a
radiological report of disease progression. During 223Ra treatment,
PSA flare phenomenon can be misinterpreted as therapeutic failure.
In contrast, a decrease in the ALP levels in responding patients is
almost always observed during treatment with 223Ra. It is important
to recognize this phenomenon in clinical practice, to avoid early
discontinuation of an ongoing and potentially effective treatment.
Bone biomarkers such as ALP should be integrated in clinical
evaluation; furthermore, morphological imaging (CT scan and
multimodal MRI) and metabolic techniques targeting bone (99mTc-
HDP WB bone scan and 18F-Fluoride PET/CT) can provide important
information.
Of note, bone scan could be confounder as PSA due to the bone
flare phenomenon that can wrongly be misinterpreted for disease
progression.
In this setting, nuclear medicine offers today different
radiopharmaceutical options for the detection of metastatic PCa.
We can divide them in two main subsets: bone targeting (i.e. 99mTcphosphonate
and 18F-Fluoride) and cancer targeting agents (11C/18FCholine,
18F-FDG, 68Ga-PSMA, 18F-FACBC, and 11C-Acetate),
although some of them are still considered as experimental and
therefore not applicable in clinical practice.
In the ALSYMPCA trial a bone scan and a CT scan were
performed to evaluate patients for enrollment; however, no imaging
modalities were scheduled during and after treatment. So how
to evaluate these patients is still matter of debate. There are no
established criteria to evaluate bone disease; in fact, RECIST criteria
consider bone metastases as non-target lesions [62]. According to the
PCWG3 criteria [63], focused on clinical evaluation, and to the St
Gallen Advanced Prostate Cancer Consensus Conference (APCCC)
[36], the clinical benefit should represent the most important criteria
of response to treatment.
Imaging and Radium-223
The role of imaging in patients candidates to 223Ra has twice
objective: a) to select appropriate subjects before to start the therapy
and b) to monitor the early and delayed evaluation of response to
treatment.
As reported by ALSYMPCA trial [20], patients without any
visceral involvement and with the lymph node metastases less
than 3 cm in diameter can be submitted to Radium-223 treatment.
More often, these criteria can be assessed by using three-phase
contrast enhancement Computed Tomography (CT). However, the
evaluation of bone involvement by CT, is scarce thus requiring more
sophisticated or specific imaging such as bone scintigraphy with 99mTcdiphosphonate.
In the last years, some papers have been published
about the role of bone scintigraphy in patients who are candidates
to 223Ra, for evaluating 1) the extension of disease, 2) the response
to treatments and 3) the hematological effects of 223Ra treatments in
patients with high load of disease [64,65]. As reported by Nome et al.
[65], the post 223Ra treatment reduced uptake of 99mTc-diphosphonate
may reflect a diminished tumour burden, as well as a direct radiation
effect on the osteoblasts (stunning or cell death) (Figure 1). However,
small or microscopic bone metastases surrounded by no or minimal
osteoblast activity, and therefore no major uptake of 223Ra, are not
sufficiently irradiated and may thus increase in size, resulting in
new sites of 99mTc-diphosphonate uptake on the post-treatment
bone scans. To overpass this latter phenomenon, the same authors
suggested to use PET/CT systems that have much higher geometric
resolution than conventional gamma cameras, and will detect smaller
lesions and therefore more lesions than conventional bone scans.
18F-Fluoride PET/CT is an alternative imaging modality for the
assessment of bone metastases in patients with metastatic prostate
cancer.
Last studies reported the advantages of 18F-Fluoride PET/CT in
patients treated with 223Ra-dichloride, because a close correlation
between the magnitude of reduction in tracer uptake and ALP was
seen [66]. Furthermore, as for bone scintigraphy, also 18F-Fluoride
PET/CT can determine which patients will benefit from 223Ra and
which will develop bone marrow failure [67]. Few data are now
available about PET/CT with 11C/18F-Choline, 68Ga-PSMA and
18F-FACBC before and after 223Ra treatment (Figure 2).
Some preliminary results demonstrated more advantages of
cancer targeting tracers (i.e. 68Ga-PSMA or 11C/18F-Choline) than
bone targeting ones (i.e. 18F-Fluoride) [22,68,69]. In fact, cancer
targeting radiopharmaceutical agents can assess both the metabolism
of cancer at bone level and in the viscera (e.g. lung, liver and lymph
nodes). Moreover, cancer targeting agents are less influenced by flare
phenomenon than bone targeting radiotracer.
The flare phenomenon, defined as an increase in the number or
intensity of bone lesions with subsequent improvement while the
patient is receiving systemic therapy, have been already reported
during 223Ra treatment for the pain (phase I trial), for the PSA levels
and for 18F-Fluoride PET/CT [66]. Nuclear imaging modalities offer
different alternatives for the evaluation of patients who are candidates
to or treated with 223Ra-dicholide, but few established data are now
available. Moreover, comparative data among imaging morphological
modalities like CT, functional imaging strategies like MRI and
metabolic imaging such as PET/CT with different radiotracers are
necessary for better understanding the advantages from sophisticated
imaging systems.
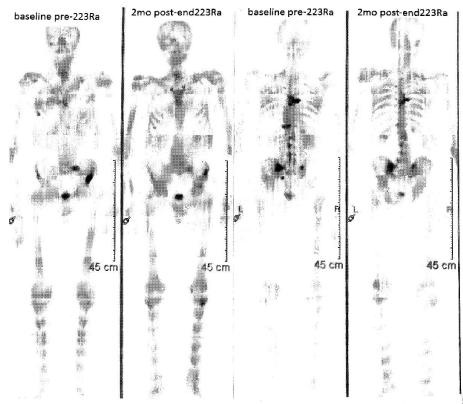
Figure 2
Figure 2
Bone scan before and after 223Ra treatment, showing a clear reaponse of mCRP lesions (anterior view on the left, posterior view on the right.
Discussion
In the last 5 years, the treatment landscape for patients with
mCRPC has rapidly changed. Besides Radium-223, new hormonal,
cytotoxic, and immunotherapeutic drugs have demonstrated
improvement in OS by randomized trials. Therefore, the identification
of the optimal treatment strategy, how to define the therapeutic
sequences and how to quantify or assess the response, in each single
patient, need to be explored.
The benefit observed with 223Ra is similar to that seen with the other
life-prolonging drugs. Most men with mCRPC are likely to receive all
these treatments, although the optimal sequencing and combinations
are matters of debate. ESMO recently validated a reproducible tool
to measure the magnitude of clinical benefit obtained from various
therapies for solid tumors. To evaluate the benefit of treatments,
outcomes like survival, quality of life, and toxicity were used [70].
Interestingly, Radium-223 was the only treatment for PCa that
has received the maximum score of 5. This score for 223Ra may be
ascribable to documented improved OS, improved time to SSEs,
better quality of life, and reduced need for hospitalization.223Ra is
often relegated to late-stage mCRPC, consistently with the use of
beta- and gamma-emitting radionuclides.
With emerging PET-imaging technologies identifying early
metastatic disease, deployment of 223Ra in the micro metastatic
setting could yield even greater clinical benefit given the smaller
amount of tumour burden [71]. Furthermore, it is important not to
leave treatment with 223Rafor the late phase of disease, to avoid the
development of visceral metastases that makes patients ineligible
for 223Ra itself. In particular, in the ALSYMPCA trial 223Ra showed
to be safe and effective irrespective of prior docetaxel use, and
post – ALSYMPCA data documented the safety of chemotherapy
administration after 223Ra.
On the other hand, the unique mechanism of action of 223Ra does
not potentially overlap with other available treatments, and the drug is
suitable for both sequencing and combination studies. Combination
therapy may improve outcomes without increasing toxicities [56].
Radium-223 is well tolerated. In the ALSYMPCA trial, there were
more adverse events in the placebo group than the radium-223 group.
Also, EAP and retreatment trials confirmed how safe and manageable
the drug is. However, patients should be evaluated for toxicities using
a complete blood count. Complete blood count should be obtained
before each cycle of Radium-223 [20], and clinical examination must
be performed at each cycle, since it remains the best instrument to
evaluate patient response and to drive physicians to further choice.
Future Perspectives
Preliminary results from an ongoing phase I/IIa trial showed
that 223Ra in combination with docetaxel in mCRPC is feasible and
safe (with lower docetaxel dose at 60 mg/mq every 3 weeks and 223Ra
for 5 cycles every 5 weeks) [71]. Currently, several clinical trials are
evaluating safety and efficacy of combination treatments of 223Ra with
abiraterone acetate (ERA 223, NCT02043678) and enzalutamide
(PEACE III, NCT02194842). Another randomized trial compared
50kBq/kg for 12 cycles versus 80 kBq/kg for 6 cycles versus standard
dose; this trial completed enrollment, data analysis is ongoing. A
potential clinical benefit 223Ra in the hormone-naïve setting is yet to
be investigated [43].
Given the mechanism of bone targeting of Radium-223, it is
likely that it will have activity against other cancers. There is some
interest in extending the treatment indications for Radium-223, with
a Phase 1/2 study in patients with osteosarcoma (NCT01833520),
a Phase 2 studies in bone predominant metastatic breast cancer
(NCT01070485) and metastatic radioiodine-refractory thyroid
cancer (NCT02390934).
Acknowledgment
The Authors are grateful to Mrs Anna Luisa De Simone Sorrentino for her valid editorial collaboration (Milano).
References
- Zustovich F, Pastorelli D. Therapeutic management of bone metastasis in prostate cancer: an update. Expert Rev Anticancer Ther. 2016; 6: 1-13.
- Gartrell BA, Saad F. Managing bone metastases and reducing skeletal related events in prostate cancer. Nat Rev Clin Oncol. 2014; 11: 335-345.
- Cathomas R, Bajory Z, Bouzid M, El Ghoneimy A, Gillessen S, Goncalves F, et al. Management of bone metastases in patients with castration-resistant prostate cancer. Urol Int. 2014; 92: 377-386.
- Butoescu V, Tombal B. Practical guide to bone health in the spectrum of advanced prostate cancer. Can J Urol. 2014; 21: 84-92.
- Broder MS, Gutierrez B, Cherepanov D, Linhares Y. Burden of skeletal-related events in prostate cancer: unmet need in pain improvement. Support Care Cancer. 2015; 23: 237-247.
- Nieder C, Haukland E, Pawinski A. Anaemia and thrombocytopenia in patients with prostate cancer and bone metastases. BMC Cancer. 2010; 10: 284.
- Beer TM, Bergenstock M, Birt K. Darbepoetin alfa administered every 4 weeks for anemia in patients with advanced prostate cancer. Clin Genitourin Cancer. 2007; 5: 329-333.
- GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012.
- Horwich A, Parker C, de Reijke T, Kataja V. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013; 24: 106-114.
- DePuy V, Anstrom KJ, Castel LD, Schulman KA, Weinfurt KP, Saad F. Effects of skeletal morbidities on longitudinal patient-reported outcomes and survival in patients with metastatic prostate cancer. Support Care Cancer. 2007; 15: 869-876.
- Halabi S, Vogelzang NJ, Kornblith AB, Ou SS, Kantoff PW, Dawson NA, et al. Pain predicts overall survival in men with metastatic castration-refractory prostate cancer. J Clin Oncol. 2008; 26: 2544-2549.
- Tannock IF, de Wit R, Berry WR. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004; 351: 1502-1512.
- Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004; 351:1513-1520.
- de Bono JS, Oudard S, Ozguroglu M. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistantprostate cancer progressing after docetaxel treatment: a randomized open-label trial. Lancet. 2010; 376: 1147-1154.
- de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. COU-AA-301 Investigators. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011; 364: 1995-2005.
- Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. COU-AA-302 Investigators. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013; 368:138-148.
- Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. AFFIRM Investigators. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012; 367: 1187-1197.
- Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. PREVAIL Investigators. Enzalutamide in Metastatic Prostate Cancer before Chemotherapy. N Engl J Med. 2014; 371: 424-433.
- Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fosså SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013; 369: 213-223.
- Renzulli JF 2nd, Collins J, Mega A. Radium-223 dichloride: illustrating the benefits of a multidisciplinary approach for patients with metastatic castration-resistant prostate cancer. J Multidiscip Healthc. 2015; 8: 279-286.
- Bombardieri E, Evangelista L, Ceresoli GL, Boccardo F. Nuclear medicine and the revolution in the modern management of castration-resistant prostate cancer patients: from (223) Ra-dichloride to new horizons for therapeutic response assessment. Eur J Nucl Med Mol Imaging. 2016; 43: 5-7.
- Hoskin P, Sartor O, O’Sullivan JM, Johannessen DC, Helle SI, Logue J, et al. Efficacy and safety of radium-223 dichloride inpatients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: aprespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol. 2014; 15: 1397-1406.
- Nilsson S, Franzén L, Parker C, Tyrrell C, Blom R, Tennvall J, et al. Two-year survival follow-up of the randomized, double-blind, placebo-controlled phase II study of radium-223 chloride in patients with castration-resistant prostate cancer and bone metastases. Clin Genitourin Cancer. 2013; 11: 20-26.
- Fizazi K, Massard C, Smith M, Rader M, Brown J, Milecki P, et al. Bone-related parameters are the main prognostic factors for overall survival in men with bone metastases from castration-resistant prostate cancer. Eur Urol. 2015; 68: 42-50.
- Jadvar H, Challa S, Quinn DI, Conti PS. One-year post approval clinical experience with radium-223 dichloride in patients with metastatic castrate-resistant prostate cancer. Cancer Biother Radiopharm. 2015; 30: 195–199.
- Etchebehere EC, Milton DR, Araujo JC, Swanston NM, Macapinlac HA, Rohren EM. Factors affecting (223) Ra therapy: clinical experience after 532 cycles from a single institution. Eur J Nucl Med Mol Imaging. 2016; 43: 8-20.
- Bruland OS, Nilsson S, Fisher DR. High-linear energy transfer irradiation targeted to skeletal metastases by the alpha-emitter 223Ra: adjuvant or alternative to conventional modalities? Clin Cancer Res. 2006; 12: 6250s-6257s.
- Henriksen G, Fisher DR, Roeske JC. Targeting of osseous sites with alpha-emitting 223Ra: comparison with the beta-emitter 89Sr in mice. J Nucl Med. 2003; 44: 252-259.
- Henriksen G, Breistøl K, Bruland ØS, Fodstad Ø, Larsen RH. Significant antitumor effect from bone-seeking, alpha-particle-emitting 223Ra demonstrated in an experimental skeletal metastases model. Cancer Res. 2002; 62: 3120-3125.
- Vogelzang NJ, Coleman RE, Michalski JM, Nilsson S, O'Sullivan JM, Parker C, et al. Hematologic Safety of Radium-223 Dichloride: Baseline Prognostic Factors Associated With Myelosuppression in the ALSYMPCA Trial. Clin Genitourin Cancer. 2016.
- Valdagni R, Albers P, Bangma C, Drudge-Coates L, Magnani T, Moynihan C, et al. The requirements of a specialist Prostate Cancer Unit: a discussion paper from the European School of Oncology. Eur J Cancer. 2011; 47: 1-7.
- Valdagni R, Van Poppel H, Aitchison M, Albers P, Berthold D, Bossi A, et al. Prostate Cancer Unit Initiative in Europe: A position paper by the European School of Oncology. Crit Rev Oncol Hematol. 2015; 95: 133-143.
- Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol. 2008; 26: 242-245.
- Oudard S. TROPIC: Phase III trial of cabazitaxel for the treatment of metastatic castration-resistant prostate cancer. Future Oncol. 2011; 7: 497-506.
- Gillessen S, Omlin A, Attard G, de Bono JS, Efstathiou E, Fizazi K, et al. Management of patients with advanced prostate cancer: recommendations of the St Gallen Advanced Prostate Cancer Consensus Conference (APCCC). Ann Oncol. 2015; 26: 1589-1604.
- Smith MR, McGovern FJ, Zietman AL, Fallon MA, Hayden DL, Schoenfeld DA, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med. 2001; 345: 948-955.
- Smith MR, Eastham J, Gleason DM, Shasha D, Tchekmedyian S, Zinner N. Randomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J Urol. 2003; 169: 2008-2012.
- Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002; 94: 1458-168.
- Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2001; 96: 879-882.
- Smith MR, Egerdie B, Hernández Toriz N, Feldman R, Tammela TL, et al. Denosumab in men receiving androgen-deprivationtherapy for prostate cancer. N Engl J Med. 2009; 361: 745-755.
- Fizazi K, Carducci M, Smith M, Damião R, Brown J, Karsh L, et al. Denosumab versus zoledronic acid for treatment of bonemetastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011; 377: 813-822.
- Wilson JM, Parker C. The safety and efficacy of radium-223 dichloride for the treatment of advanced prostate cancer. Expert Rev Anticancer Ther. 2016; 16: 911-918.
- D'Angelo G, Sciuto R, Salvatori M, Sperduti I, Mantini G, Maini CL, et al. Targeted “bone-seeking” radiopharmaceuticals for palliative treatment of bone metastases: a systematic review and meta-analysis. Q J Nucl Med Mol Imaging. 2012; 56: 538-543.
- Collins C, Eary JF, Donaldson G, Vernon C, Bush NE, Petersdorf S, et al. Samarium-153-EDTMP in bone metastases of hormone refractory prostate carcinoma: a phase I/II trial. J Nucl Med.1993; 34: 1839-1844.
- Porter AT, McEwan AJ, Powe JE, Reid R, McGowan DG, Lukka H, et al. Results of a randomized phase-III trial to evaluate the efficacy of strontium-89 adjuvant to local field external beam irradiation in the management of endocrine resistant metastatic prostate cancer. Int J Radiat Oncol Biol Phys. 1993; 25: 805-813.
- Metastron. Available US Food and Drug Administration. US Department of Health and Human Services.
- SartorO, Reid RH, Hoskin PJ, Quick DP, Ell PJ, Coleman RE, et al. Samarium-153-Lexidronam complex for treatment of painful bone metastases in hormone-refractory prostate cancer. Urology. 2004; 63: 940-945.
- James ND, Pirrie S, Barton D, Brown JE, Billingham L, Collins SI, et al. Clinical outcomes in patients with castrate-refractory prostate cancer (CRPC) metastatic to bone randomized in the factorial TRAPEZE trial to docetaxel (D) with strontium-89 (Sr89), zoledronic acid (ZA), neither, or both (ISRCTN 12808747). J Clin Oncol. 2013; 31.
- Fizazi K, Lipton A, Mariette X, Body JJ, RahimY, Gralow JR, et al. Randomized phase II trial of denosumab in patients with bone metastases fromprostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol. 2009; 27: 1564-1571.
- Heron DE, Brufsky A, Beriwal S, Kurman M. Myelotoxicity of samarium Sm 153 lexidronam in patients receiving prior treatmentwith chemotherapy or radiotherapy. Ann Oncol. 2008; 19: 1639-1643.
- Nilsson S, Larsen RH, Fosså SD, Balteskard L, Borch KW, Westlin JE, et al. First clinical experience with alpha-emitting radium-223 in the treatment of skeletal metastases. Clin Cancer Res. 2005; 11: 4451-4459.
- Chittenden SJ, Hindorf C, Parker CC, Lewington VJ, Pratt BE, Johnson B, et al. A Phase 1, Open-Label Study of the Biodistribution,Pharmacokinetics, and Dosimetry of 223Ra-Dichloride in Patients with Hormone-Refractory ProstateCancer and Skeletal Metastases. J Nucl Med. 2015; 56: 1304-1309.
- Dauer LT, Williamson MJ, Humm J, O'Donoghue J, Ghani R, Awadallah R, et al. Radiation safety considerations for the use of 223Ra Cl2 DE in men with castration-resistant prostate cancer. Health Phys. 2014; 106: 494-504.
- Sartor O, Coleman R, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol. 2014; 15: 738-746.
- Saad F, Carles J, Gillessen S, Heidenreich A, Heinrich D, Gratt J, et al. Radium-223 International Early Access Program Investigators. Radium-223 and concomitant therapies in patients with metastatic castration-resistant prostate cancer: an international, early access, open-label, single-arm phase 3b trial. Lancet Oncol. 2016; 17: 1306-1316.
- National Comprehensive Cancer Network. 2015.
- Urogenital Cancers. ESMO pocket guidelines & mobile app.
- Sartor O, Hoskin P, Coleman RE, Nilsson S, Vogelzang NJ, Petrenciuc O, et al. Chemotherapy following radium-223 dichloride treatment in ALSYMPCA. Prostate. 2016; 76: 905-916.
- Sartor O, Heinrich D, Mariados N, Méndez Vidal MJ, Keizman D, et al. Radium-223 (Ra-223) Re-treatment (Re-tx): First Experience From an International, Multicenter, Prospective Study in Patients (Pts) With Castration-Resistant Prostate Cancer and Bone Metastases (mCRPC). 2016.
- De Vincentis G, Follacchio GA, Frantellizzi V, Liberatore M, Monteleone F, Cortesi E. Prostate-Specific Antigen Flare Phenomenon During 223Ra-Dichloride Treatment for Bone Metastatic Castration-Resistant Prostate Cancer: A Case Report. Clin Genitourin Cancer. 2016; 14: e529-e533.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45: 228-247.
- Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Prostate Cancer Clinical Trials Working Group 3.Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations From the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016; 34: 1402-1418.
- Miederer M, Thomas C, Beck J, Hampel C, Krieger C, Baqué PE, et al. Haematopoietic toxicity of radium-223 in patients with high skeletal tumour burden. Nuklearmedizin. 2015; 54: 197-203.
- Nome R, Hernes E, Bogsrud TV, Bjøro T, Fosså SD. Changes in prostate-specific antigen, markers of bone metabolism and bone scans after treatment with radium-223. Scand J Urol. 2015; 49: 211-217.
- Cook G Jr, Parker C, Chua S, Johnson B, Aksnes AK, Lewington VJ. 18F-fluoride PET: changes in uptake as a method to assess response in bone metastases from castrate-resistant prostate cancer patients treated with 223Ra-chloride (Alpharadin). EJNMMI Res. 2011; 7: 1: 4.
- Etchebehere EC, Araujo JC, Milton DR, Erwin WD, Wendt RE 3rd, Swanston NM, et al. Skeletal Tumor Burden on Baseline 18F-Fluoride PET/CT Predicts Bone Marrow Failure After 223Ra Therapy. Clin Nucl Med. 2016; 41: 268-273.
- Evangelista L, Bertoldo F, Boccardo F, Conti G, Menchi I, Mungai F, et al. Diagnostic imaging to detect and evaluate response to therapy in bone metastases from prostate cancer: current modalities and new horizons. Eur J Nucl Med Mol Imaging. 2016; 43: 1546-1562.
- Uprimny C, Kroiss A, Nilica B, Buxbaum S, Decristoforo C, Horninger W, et al. (68)Ga-PSMA ligand PET versus (18)F-NaF PET: evaluation of response to (223)Ra therapy in a prostate cancer patient. Eur J Nucl Med Mol Imaging. 2015; 42: 362-363.
- Cherny NI, Sullivan R, Dafni U, Kerst JM, Sobrero A, ZielinskiC, et al. generic validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies : The European Society for Medical Oncology Magnitude of Clinical Benefit Scale ( ESMO-MCBS). Ann Oncol. 2015; 26: 1547-1573.
- Madan RA, Gulley JL, Dahut WL. Radium-223 in prostate cancer: emitting the right signals. Lancet Oncol. 2016; 17: 1186-1187.
- Morris MJ, Hammers HJ, Sweeney C, Antonarakis ES, Cho SY. Safety of radium-223 dichloride (Ra-223) with docetaxel (D) in patients with bone metastases from castration-resistant prostate cancer (CRPC): a phase I Prostate Cancer Clinical Trials Consortium Study. ASCO Meeting Abstracts. 2013; 31: 5021.