Case Report
Excellent Response to Dual Her2 Targeted Therapy in a Patient with Advanced Colorectal Cancer in Fourth Line of Treatment
Juárez A1*, Montero M1, Llorente M1, Gálvez E1, Durán R2 and Llorca C1
1Department of Medical Oncology, Elda Hospital, Spain
2Department of Pathological Anatomy, Elda Hospital, Spain
*Corresponding author: Juárez A, Department of Medical Oncology, Elda Hospital, Spain
Published: 06 Dec, 2016
Cite this article as: Juárez A, Montero M, Llorente M,
Gálvez E, Durán R, Llorca C. Excellent
Response to Dual Her2 Targeted
Therapy in a Patient with Advanced
Colorectal Cancer in Fourth Line of
Treatment. Clin Oncol. 2016; 1: 1160.
Abstract
Men 47 years old with staged IV colon cancer Ras wild type and her 23+, who had progressed
to all approved treatments, get an spectacular response with dual her2 targeted Therapy without
chemotherapy. This is the first case reported with this treatment, supporting the results of
HERACLES study, as well as encouraging the development of phase III studies.
Keywords: Colon cáncer; Her2; Trastuzumab; Lapatinib
Introduction
Her2 neuis an important oncogen in breast and gastric cancer, but its prevalence and significance
in Colorectal cancer (CRC) is poorly documented.
Any studies have shown her2 overexpression rates so low (3.8%), at least in therapeutic
ranges (2+ o 3+ for IHC), than have not justified the developement of this marker to therapeutic
management of Colorectal cancer [1].
Case Presentation
Male 45 years old, with unique history of hypertension well controlled.
He was diagnosed on January 2014 of a sigma adenocarcinoma stage IV, with liver metastases.
Valued in multidisciplanary committe, with asymptomatic primary and irresecab leliver metastasis,
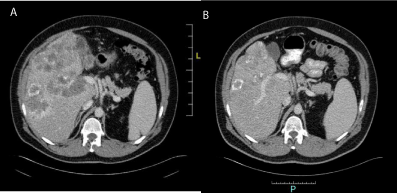
began systemic treatment with palliative chemotherapy (Figure 1A and B).
He Received first line treatment into clinical trial VISNU [2] after molecular study (native Ras,
native PI3K, native Braf and 10 circulating tumor cells), randomized to FOLFOX-BEVACIZUMAB.
After 17 cicles, with best response of partial response after 6 cicles, we stopped treatment for disease
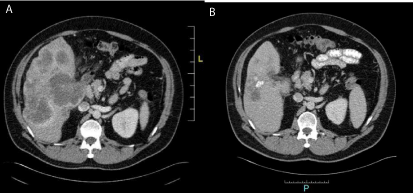
progression in november 2014 (Figure 2A and B). We changed treatment to FOLFIRI-CETUXIMAB,
begining on 24.11.14, with best response of partial response documented in first reevaluation after
sixth cicle, and suspending treatment due to progression after twelfth.
On june 2015, began third line treatment with regorafenib, with liver progresion in first
reevaluation on september 2015.
The Patient maintained Excellent overall status, but needed morfine for abdominal pain and in
october began with tumoral fever controlled with anti-inflamatory (naproxene).
On december 2015, with demonstrated her2 overexpression (IHC 3+), and supported for
results of HERACLES study presented in ASCO 2015 [3], the Patient began treatment with dual
her2 targeted therapy. Received trastuzumab 2mg/kg (first dose 4mg/kg) and lapatinib 1000mg/
day. The Patient had excellent tolerance, with trombopenia G2 at the beginning, resolved with one
week break, and rash grade 1. The Patient had significative clinical benefit, stopping naproxene and
morphine, and with analytic and radiologic response after third and sixth cicle of treatment.
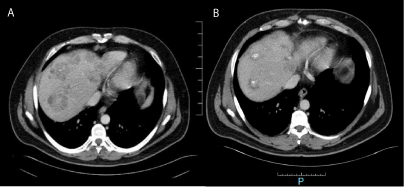
In june 2016, Patient began with pruritus secondary to colestasis, confirming tumoral
progression in TC-scan, with a progression free survival of 6 months. The Patient died in august
2016 (Figure 3A and B).
Figures
Figures
Discussion
Use of Her2-targeted therapy added to standard chemotherapy
forme tastatic colorectal cáncer, was failed in two clinicaltrials, due to
lack of efficacy [4] or insufficient accrual [5].
More recent studies have shown, nevertheless, higher over
expression rates, until 10%, confirming this overexpression, both in
the primary tumor and the metastasis (suggesting that overexpression
is an early molecular alteration that persist during tumour
progression). It is documented her2 over expression independent to
kras status, finding her2 overexpression in therapeutic range in 5.3%
of patients with mutated kras and 7.5% of patients with native kras
[6].
Pre clinical studies show her2 overexpression in 7% of colorectal
patients, further showing in cancer patients-derived xenografts
that HER2 activating mutations in to colorectalcancer celllines
produced resistance to anti-EGFR therapy by sustaining MAPK
phosphorylation. In this study, treatment with a single HER2 targeted
drug (trastuzumab, neratinib, orlapatinib) delayed tumor growth, but
dual HER2 targeted therapy with trastuzumab plus tyrosine kinase
inhibitors produced regression of these HER2 mutated PDX’s [7].
On this basis, it was designed HERACLES study [8], with
two investigation alarms: trastuzumab + lapatinib (Arm A) and
Trastuzumab + Pertuzumab (arm B, pending results).
The arm A results were presented in ASCO 2015. Patients
progressing after fluoropyrimidines, oxaliplatin, irinotecan,
cetuximab or panitumumab were eligible if tumor was HER2+
[IHC3+ or 2+ and FISH positive (HER2:CEP17 >2) in > 50% cells
and kraswt. It is allowed previous treatment with bevacizumab,
aflibercept or regorafenib. All patients receive-lapatinib 1000 mg
daily and trastuzumab 4 mg/kg iv load, followed by 2 mg/kg iv weekly.
Primary end point was Objective Response Rate according to RECIST 1.1 criteria. Secondary end points are description of the
frequency and severity of Adverse Events and PFS.
From 849 patients with mCRC KRAS exon 2 wild-type patients,
46 (5.4%) were HER2-positive. 24 patients were enrolled but 23 were
evaluable for response. 18 patients had HER2 IHC 3+ tumours and 6
patients had HER2 IHC 2+ tumours. In 83% of patients, the primary
site of tumour was left colon. Median number of prior lines of therapy
was 5 (range 2-8) with 83% of patients who received more than 3
prior therapy regimens. All patients were treated previously with
cetuximab or panitumumab. How ever, previous response to EGFR
monoclonal antibodies was 0%.
The study treatment was well tolerated, with no grade 4-5
toxicities. The compliance was good with 96,4% of dosage received as
planned. There was no patient off-treatment due to toxicities.
The primary endpoint was met with 8 out of 23 objective
responses in patients heavily pretreated with standard therapies. The
response rate (partial response plus complete response) was 34,7%.
Stable disease of at least 4 months was recorded in 30,4% of patients.
The disease control rate was 78%.
Median TTP was 5,5 months. The TTP by HER2 score was 7,3
months in patients with HER2 3+ and 4,2 months in HER2 2+.
The response and tolerance obtained in our case, according to
results of phase II trial HERACLES, encourage to continue with
the development of her2 targeted therapy in CCR cancer (Table 1).
Probablyits use in early lines of treatment could improve these results,
justified fortheaparent anti-EGFR resistence observed in preclinical
studies [7] and HERACLES trial [8].
Besides, the Won-Sukstudy motivate the her2 analysis in CCR
independently of RAS mutation [6].
If we consider that at least5% of patients may have an Her2
overexpression, according to CCR prevalence, it would be a
therapeutic option to approximately 60000patientseveryyear, higher
than the number of patients who may benefit From other targeted
treatments like ALK inhibitors in lung cancer.
The drugs optimization, specially targeted treatments, with a known predictive marker, is critical. The optimal cancer treatment
goes in the way to selection treatment according to molecular markers
rather than anatomic or clinical features.
Declaration of interests the authors certify that they have NO
affiliations with or involvement in any organization or entity with
any financial interest, or non-financial interest in the subject matter
or materials discussed in this manuscript.
Figures
Table 1
References
- Schuell B, Gruenberger T, Scheithauer W, Zielinski Ch, Wrba F. HER 2/neu protein expression in colorectal cancer. BMC Cancer 2006; 6: 123.
- VISNU 1: Ensayo clínico fase III aleatorizado, abierto y multicéntrico, para evaluar la eficacia de FOLFOLX+BEVACIZUMAB vs. FOLFOXIRI+BEVACIZUMAB como tratamien to de primera línea de pacientes con CCRm no tratado previamente, con 3 o más CTC. Promotor: TTD.
- Siena S, Sartore-Bianchi A, Lonardi S, Trusolino L, Martino C, Bencardino, et al. Trastuzumab and lapatinib in HER2-amplified metastatic colorectal cancer patients (mCRC): The HERACLES trial. J Clin Oncol. 2015; 33: 3508.
- Cark JW, Niedwiecki D, Hollis D. Phase II trial of 5-fluoracil, leucovorin, oxaliplatin and trastuzumab for patients with metastatic colorectal cancer refractory to initial therapy. Proc Am Soc Clin. 2003; 21: 3584.
- Ramanathan RK, Hwang JJ, Zambomi, Sinicrope FA, Safran H, Wong MK, et al. Low overexpression of Her-2/neu in advanced colorectal cancer limits tje usefulness of trastuzumab (herceptin) and irinotecan as therapy. Cancer Invest. 2004; 22: 858-865.
- Lee WS, Park YH, Lee JN, Baek JH, Lee TH, Ha SY. Comparison of HER2 expression between primary colorectal cancer and their corresponding metastases. Cancer Medicine. 2014; 3: 674-680.
- Kavuri, S, Jain N, Galim F, Cottino F, Leto SM, Migliardi G, et al. HER2 Activating Mutations Are Targets for Colorectal Cancer Treatment. Cancer Discov. 2015; 5: 832–841.
- Sartore-Bianchi A, Trusolino L, Martino C, Bencardino K, Lonardi S, Bergamo F, et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016; 17: 738-746.