Research Article
Genistein Induced Apoptosis of Gastric Cancer Cell through Bcl-2 and Caspase-3 Regulation
Yusheng Li1#, Wei Luo1#, Guangxin Li2, Qing Liu1, Yanping Liu3* and Lichun Sun4*
1Department of Orthopedics, Xiangya Hospital, Central South University, China
2Department of Pathology, Chong Qing Cancer Institute, China
3Department of cell Biology, School of life Sciences, Central South University, China
4Department of Medicine, Tulane University Health Science Center, USA
#Equally Contributed
*Corresponding author: Lichun Sun, Department of Medicine, Tulane University Health Science Center, New Orleans, LA 70112, USA
Published: 30 Nov, 2016
Cite this article as: Li Y, Luo W, Li G, Liu Q, Liu Y, Sun
L. Genistein Induced Apoptosis of
Gastric Cancer Cell through Bcl-2 and
Caspase-3 Regulation. Clin Oncol.
2016; 1: 1150.
Abstract
Aim: To investigate the effects of genistein on cell apoptosis in the human gastric cancer cell BGC-
823 and the potential regulation pathway involved in its apoptotic effect.
Methods: Effects of genistein on the proliferation, cell cycle progression and apoptosis of gastric
cancer cells were examined in vitro. Western blotting were applied to analyze the proteins associated
with the biological effects of genistein.
Results: Gastric cancer cell growth was attenuated by genistein treatment in dose- and timedependent
manner. Cell cycle progression analysis showed that apoptosis was enhanced in genistein
treated cells and cell cycle arrest at G2/M phase. Increased apoptosis was accompanied by increased
level of caspase-3 protein and decreased level of Bcl-2 protein.
Conclusions: Genistein induce the apoptosis of BGC-823 in dose- and time-dependent manner.
This apoptosis may be mediated by down-regulating the apoptosis-associated Bcl-2 gene and upregulating
the expression of apoptosis-associated caspase-3 gene. As genistein was effective in vitro
in promotion of apoptosis of gastric cancer cell by caspase-3 and Bcl-2 related apoptotic pathway, it
might be a potential therapeutic agent against gastric cancer.
Keywords: Genistein; Human gastric cancer; Apoptosis; Caspase-3; Bcl-2
Introduction
Gastric cancer, a daunting global problem, which accounts for 8% of the total number of cases
of cancer and 10% of annual deaths from cancer, is the fourth leading cause of cancer-related death
worldwide [1,2]. Despite substantial and accelerated research in the area of gastric cancer, it still
remains the third most common cancer and the second most common cause of cancer mortality
in Asia [3,4]. Until now, surgical resection has remained the frontline treatment for patients with
early stage gastric cancer. Nevertheless, the majority of such patients have poor prognosis due to
high rates of tumor recurrence as well as Lymph Node (LN) and systemic metastases. Therefore,
there is a need to develop novel therapeutic approaches to improve the outcomes of patients with
gastric cancer.
For centuries, nature has proven as a rich source of lead compounds for treatment of various
ailments [5]. Genistein, a natural isoflavone extracted from soybean products, exerts varieties of
biological functions including inhibiting microsomal lipid peroxidation, angiogenesis and inducing
differentiation of numerous cell types [6-9]. It was first discovered that genistein was a potent
inhibitor of the tyrosine-specific protein kinase activity of the epidermal growth factor receptor
in 1987 [6]. Its structure is similar to that of human 17-β-estradiol causing estrogenic and/or antiestrogenic
effects [10]. Since then, numerous researchers have studied the possible use of genistein
as a cancer chemopreventive agent based on the key role of protein tyrosine kinase inhibitors in
cancer cell growth and apoptosis [11,12]. Recently in vitro studies conducted in different cancer
cell lines confirmed that by affecting various cellular targets, genistein treatment repressed cancer
cell proliferation, induced apoptosis and led to cell cycle arrest [13-16]. A survey of recent related
literature [17] reveals that genistein might be related to induce the apoptosis of gastric cancer, but
the precise mechanism of anti-tumor activity remains unknown.
Apoptosis, also described as programmed cell death, is a highly
programmed cell death process. Selectively inducing apoptosis
in cancer cells has been increasingly recognized as a promising
therapeutic approach for many cancers. Two principal apoptosis
signal transduction pathways, which were described as the intrinsic
pathway (also known as the mitochondrial pathway) and extrinsic
pathway (also known as the death receptor pathway), have been
delineated that constitute the basic machinery for triggering apoptosis
in mammalian cells [18]. Apoptosis is controlled by the Bcl-2 family
of proteins and by Caspases, a family of cysteine proteases [19].
Apoptosis induced by these molecules can prevent carcinogenesis by
eliminating damaged cells or inhibiting abnormal cell development.
Therefore, the induction of cell cycle arrest and programmed
cell death play crucial roles in the anticancer properties of many
anticancer agents. Based on the researches above, we hypothesized
that genistein might induce the apoptosis of the human gastric cancer
cell BGC-823 through Bcl-2 and Caspase-3 regulation.
In the present study, we evaluated the effects of genistein on cell
apoptosis in the human gastric cancer cell BGC-823. We also assessed
the potential regulation pathway involved in its apoptotic effect.
Material and Methods
Cell lines and reagents
BGC-823, the human gastric cancer cell line, was provided by
Cell Culture Stock Center of Central South University. BGC-823 cells
were cultured in RPMI1640 medium (Gibco, Grand Island, NY, USA)
containing 15% fetal bovine serum (Invitrogen Life Technologies,
Carlsbad, CA, USA), penicillin 100 U/ml, stretomycin 100 μg/ml in
an atmosphere of 5% CO2 and 95% humidified air at 37°C.
Genistein ( >99% pure), was purchased from Senfu Biotechnology
Co. (Xi’an, China). Genistein was first resolved in DMSO (Fisher
Chemicals, Fair Lawn, NJ) to prepare 160 mmol/L store solution and
then serially diluted to serials of concentration prior to experiments.
Measurement of cell viability
MTT assay was used to evaluate the cell viability according to the
manufacturer's instructions. Briefly, 1×104 cells per well were plated
into 96-well plates and incubated for 3 h. The cells were subsequently
exposed to different concentrations of genistein (10, 20, 40, 60, 80 and
160 μmol/L) for 48 h. Each concentration was performed in triplicate.
MTT reagent (Promega, Madison, WI) was added. After incubation
for 4 h at 37°c, the absorbance, which is directly proportional to the
number of viable cells in cultures, was measured at 570 nm by an
automatic microplate reader (Epoch; Bio-Tek Instruments, Winooski,
VT). The cell viability was expressed as a percentage value of control
cells cultured with medium alone. The test was tripled and inhibition
rate was calculated with formula:
Inhibition rate=1-(TreatmentA570-BlankA570)/(ControlA570-
BlankA570) ×100% to produce inhibition curve and read out IC50 of
genistein.
Flow cytometry for detection of cell cycle
Cells were plated in 35 mm dishes at concentrations determined to
yield 60–70% confluence within 48 h and then treated with genistein
in indicated concentrations (10, 20, 40, 60, 80 and 160 μmol/L) for 48
h. Both the adherent and floating cells were harvested, and the cells
were resuspended in PBS, fixed with 70% ethanol at -20 overnight.
The cells were first incubated with RNase A (20 U/mL, Sigma Co.
St. Louis, MO) at 37 for 30 min and then labeled with propidium iodide (PI, Becton-Dickinson, San Diego, CA) and incubated at room
temperature in the dark for 30 min. DNA content was then analyzed
using a FACS can instrument equipped with FACS tation running
cell Quest software (Becton-Dickinson). At least 10,000 cells were
collected for each measurement in a triplicate experiment.
Flow cytometry for detection of apoptosis
BGC-823 cells were seeded in 24-well plates. After 24 h, the
medium was changed and chemicals were added with indicated
concentrations. After treatment for 48 h, all the adherent cells were
collected with 0.05% trypsin, including the floating cells in the
medium. Annexin-V (FITC) and propidium iodide (PI, Becton-
Dickinson, San Diego, CA) were used for staining according to
the manufacturer's instructions. Vehicle-treated cells were set for
control. The double-stained cells were subsequently analyzed by a
FACS Can to flow cytometer (Becton-Dickinson, Mountain View,
CA). All experiments were processed independently three times. At
least 10,000 cells were counted each time.
Caspase activity measurement
Caspase activities were measured by colorimetric assay (BioVision,
Inc., Mountain View, California, US), according to the manufacture’s
recommendations. Briefly, BGC-823 cells, treated with genistein
(10, 20, 40, 60, 80 mM) for 24 h, were harvested and incubated in
ice-cold cell lysis buffer for 30 min on ice. The supernatants were
collected and protein concentrations were determined using BCA
Protein Assay Reagent (Pierce, Rockford, IL, US). Equivalent amount
of proteins for each sample was incubated with interested caspase-9.
After incubation at 37°c for 4 h, the protease activity was determined
at 405 nm with microplate spectrophotometer (Bio-Tek Instruments,
Inc., Winooski, US).
Western blotting analysis
Western blot analysis to assess Bcl-2, Caspase-3 and Caspase-9
expression was performed as previously described [20]. Briefly, A
total of 106 cells were sedimented and lysed for 15 minutes in icecold
lyses buffer (0.1% SDS, 1% NP-40, 50mM HEPES, pH 7.4,
2mM EDTA, 100mMNaCl, 5mM sodium orthovanadate, 40 μM
p-nitrophenyl phosphate, and 1% protease inhibitor mixture set
I; Calbiochem). After removing the cell debris by centrifugation at
16200 g for 15 minutes, equal amounts of proteins were separated
on a 12% SDS-PAGE, blotted onto a nitrocellulose membrane (GE
Healthcare, Little Chalfont, United Kingdom) and blocked with
5% nonfat dry milk in PBS/Tween (0.05% Tween-20 in PBS). The
following antibodies were used: Bcl-2 antibody (Santa Cruz, CA,
USA), Caspase-3 antibody (Santa Cruz, CA, USA), Casepase-9
antibody (Santa Cruz, CA, USA). The membranes were then
incubated with the appropriate horseradish peroxidase-conjugated
secondary antibodies (1:2000). The immunoreactions were developed
by Enhanced Chemiluminescence (ECL). β-actin expression was used
as the loading control. The intensity of the specific immunoreactive
bands was detected by Super Signal West Pico Substrate (Thermopierce,
Rockford, IL) and quantified by densitometry using ImageJ
1.45 software. Data were supplied as a ratio of the β-actin for analyzing
and plotting.
Statistical analysis
All the experiments were carried out in triplicate and data are
presented as mean ± SD. Statistical analysis was performed using
SPSS software (Release 17.0, SPSS Inc.). The difference between two
groups was analyzed by the Student’s t-test. A value of p <0.05 was
considered as statistical significance.
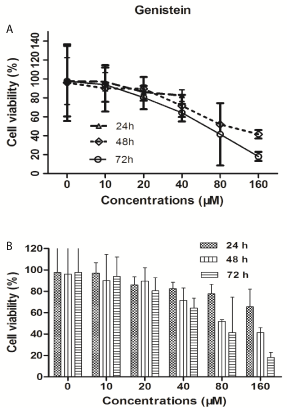
Figure 1
Results
Genistein inhibits BGC-823 cell proliferation
To examine the growth inhibitory effect of genistein, BGC-823
cells were treated with different concentrations (10, 20, 40, 60, 80 and
160 μM) of genistein for different periods of time (24, 48 and 72 h).
MTT assay for cell viability was performed. As showed in (Figure
1), the cell viability was dose-dependently reduced in gastric cancer
cell BGC-823 after genistein treatment at indicated concentrations
in a time-dependent manner. The results of MTT assay showed that
the significant suppression of BGC-823 was induced by genistein
treatment with concentration of 40 μM above after 48 h. The genistein
respectively inhibited BGC-823 cells with IC50 value of 247.3 μmol/L
for 24 h, 84.2 μmol/L for 48 h and 65.3 μmol/L for 72 h.
Genistein promotes apoptosis and induces cell cycle
arrest in BGC-823 cells
Since cell viability reduction appeared in the MTT assay, we
further detected the possibility of apoptosis occurrence in genisteintreated
BGC-823 cells using flow cytometry. BGC-823 cells were
treated with different concentrations (10, 20, 40, 60, 80 and 160
μM) of genistein for 48 h. Apoptotic cells were determined by flow cytometry using Annexin V/Propidium Iodide (PI) double labeling.
The Annexin V stain assay, an event typically associated with
apoptosis, was used to evaluate phosphatidylserine externalization
from the inner to the outer lipid layer of the plasma membrane [20].
As is described in (Table 1), the ratio of the apoptotic cells (Annexin
V-FITC-positive) in BGC-823 was significantly increased in a dosedependent
manner when treated with genistein. We also examined
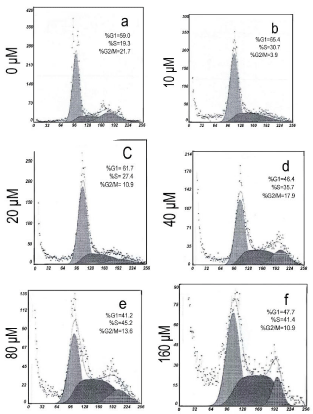
the effect of genstein on the cell cycle progression of BGC-823. As
is showed in (Figure 2), cell cycle analysis showed that exposure to
genistein (10 μM) for 48 h induced a significant proportion of cells
accumulated at the G2/M phase.
Inhibition of Bcl-2 protein enhances the apoptosisinducing
activity of genistein
Based on the results above, we further investigated the potential
effects of genistein on gastric cancer cell. Since Bcl-2 protein served
as the key player in the death receptor pathway of apoptosis [21],
we investigated the effects of genistein on this regulatory protein.
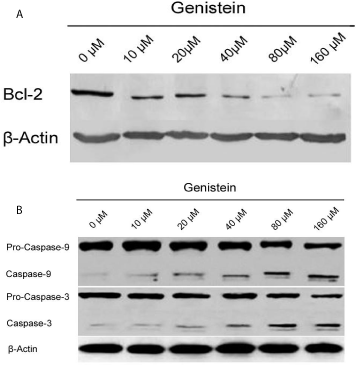
We observed that genistein decreased Bcl-2 expression in gastric
cancer cells in Western blotting assay (Figure 3A), which suggest that
genistein promotes gastric cancer cells apoptosis via Bcl-2 protein
regulating pathway. As the caspases cascade has been demonstrated to
be involved in apoptosis signal transduction and execution [7,14,22],
we further examined the expression of apoptotic protein caspase-3
and caspase-9 in this study. It showed that caspase-3 and caspase-9
expression were increased in Western blotting assay by different
concentration of genistein possessed (Figure 3B). This suggested
that genistein induce the gastric cancer cell apoptosis by activing
the caspase-3. From all the results above, caspase-3 might correlate
negatively with Bcl-2 protein in the apoptosis of BGC-83 cell.
Inhibition of caspase-3 activity upregulate the expression
of Bcl-2 protein
Caspase-3 represents one of the key proteases responsible of
cleavage of PARP and subsequent apoptosis [23,24]. It has been
reported that caspase-3 can inactivate the Bcl-2 during the apoptosis
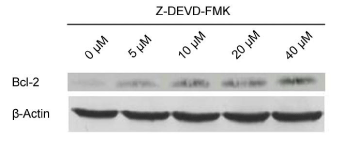
[25,26]. To further evaluate the correlation of caspase-3 and Bcl-2
protein in the apoptosis of gastric cancer cell, we use a general and
potent inhibitor of caspase-3, Z-DEVD-FMK. As showed in (Figure
4), Bcl-2 expression was significantly increased by Z-DEVD-FMK,
indicating that caspase-3 was involved in the inactivation of Bcl-2
protein during the apoptosis of BGC-823 cell.
Inhibition of Bcl-2 protein activity upregulate the
expression of caspase-9
The Bcl-2 protein probably serves to prevent the activation of
the caspase subfamily in response to apoptotic stimuli. To further
investigate whether the Bcl-2 is involved in the inactivation of
caspase-9 in the gastric cancer cell, we use a general inhibitor of
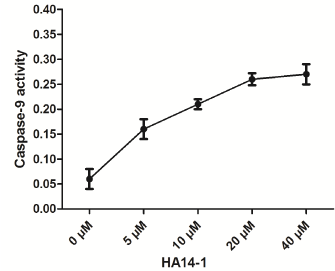
Bcl-2, HA14-1. As is shown in (Figure 5), caspase-9 expression was
significantly increased by HA14-1, indicating that Bcl-2 protein was involved in the inactivation of caspase-9 in the apoptosis of BGC-823
cell.
Table 1
Figure 2
Figure 2
Effect of genistein on cell cycle arrest in BGC-823 cells.
Flowcytometry analysis indicated a G2/M phase arrest in the BGC-823 cells
after genistein (10 μM ) treatment for 48 h. (A)0μM; (B)10μM; (C)20μM;
(D)40μM; (E)80μM; (F)160μM.
Figure 3
Figure 3
Western blotting analysis of bcl-2, caspases-3 and caspases-9
expression in different concentrated genistein treatment BGC-823 cell lines.
A) Western blotting indicated that genistein decreased bcl-2 expression
in gastric cancer cells. B) Western blotting indicated that caspase-3 and
caspase-9 expression were increased by different concentration of genistein
possessed BGC-823 cell lines.
Figure 4
Figure 4
Effect of caspase-3-specific inhibitor, Z-DEVD-FMK, on upregulation
of bcl-2 expression on BGC-823 cells.
Western blotting indicated that bcl-2 expression was significantly increased
by Z-DEVD-FMK.
Figure 5
Figure 5
Effect of bcl-2 inhibitor, HA14-1 on up-regulation of caspase-9
expression on BGC-823 cells.
Western blotting indicated that caspase-9 expression was significantly
increased by HA14-1.
Discussion
Epidemiologic studies indicate that the consumption of soycontaining
diets is associated with a lower incidence of many human
tumors and isoflavones have been proven to be the major active
components in soybased diets [27,28]. Genistein is proved to be one
of the most active flavonoids in soybeans. Accumulating evidence
suggests that genistein alters a variety of biological processes in
estrogen-related malignancies, such as hepatocellular carcinoma,
colon carcinoma, breast and prostate cancers [8,9,28,29]. Furthermore,
genistein has also been shown to induce a wide spectrum of cancer
cell apoptosis [16,30,31]. However, the molecular mechanism of
genistein in the prevention of gastric cancer remains unclear. This
study presents data showing that genistein, one of the most active
flavonoids, attenuated intro proliferation of human gastric cancer
BGC-823 cells by inducing the apoptosis and this apoptosis might
be mediated by down-regulating the apoptosis-associated Bcl-2 gene
and up-regulating the expression of apoptosis-associated caspase-3
gene. These results imply that an adjuvant therapy with genistein may
have potential therapeutic benefits in gastric cancer.
Our results suggest that the enhanced apoptosis and suppressed
cell cycle progression may be the mechanisms of action of genistein
on gastric cancer BGC-823 cell line. These results are consistent with
the previous reports on other tumor cell lines [14-16,30]. HB Zhou
et al reported that genistein inhibited cell proliferation and induced
apoptosis in primary gastric cancer cells and that these events were
related to the down-regulating the apoptosis-associated bcl-2 gene and
up-regulating the expression of apoptosis-associated Bax genes [17].
As a radical regulator of apoptosis, Bcl-2, which is known to regulate
apoptotic pathways and protect against cell death, is negatively
related to malignant level and prognosis of gastric cancer patients
[32]. It is encouraging that, consistent with previous studies, we have
demonstrated that genistein exerted anticancer effects partially in
a Bcl-2 dependent manner. Furthermore, genistein arrested BGC-
823 cells at the G2/M phase in a dose-dependent manner. This was
correlated to its effect of triggering the downregulation of Bcl-2. And
these results were further substantiated by the accompanied increases in the expression of the caspases proteins.
Caspases(Cysteine Aspartate Specific Proteases) are a group
of cysteine proteolytic enzymes produced by the cells of living
organisms [33]. With regard to the length of the prodomain, caspases
are divided into two subfamilies: Those with a long prodomain
are pro-inflammatory and apoptosis initiators; and those with a
short prodomain are “executioner caspases”. During apoptosis,
several effector proteases such as caspase-3 mediate the deliberate
disassembly of the cell into apoptotic bodies. These downstream
caspases are activated through proteolytic cleavage by either caspase-8
or caspase-9, two upstream initiator caspases. A survey of researches
favour the concept that the Bcl-2 family proteins function upstream
of caspase activation and prevents apoptosis by suppressing caspase
activity. It is now widely accepted that Bcl-2 functions to prevent
the activation of caspases implicated in the execution phase of
apoptosis (caspase-3-like enzymes) rather than being attacked and/or
inactivated by these proteases [22,25,34,35]. Several lines of evidences
show that Bcl-2 family proteins are involved in controlling the
release of cytochrome c from the mitochondria to activate caspase-9
[34,36,37]. Others reported that caspase-3, as a major apoptotic
executor, can eliminate Bcl-2 protein in vitro [38,39]. However, the
biochemical connection between the Bcl-2 and caspases has been
elusive, especially in gastric cancer.
Indeed, our analysis on between caspase-3 inactivation and Bcl-2
expression following applying Z-DEVD-FMK in gastric cancer cell
showed that Bcl-2 expression was significantly increased by Z-DEVDFMK,
indicating that caspase-3 was involved in the inactivation of
Bcl-2 protein during the apoptosis of BGC-823 cell. Furthermore
from the results of the present study, caspase-9 expression was
significantly increased by HA14-1, indicating that Bcl-2 protein was
involved in the inactivation of caspase-9 in the apoptosis of BGC-823
cell. All the results above imply that Bcl-2 might not only inactivate
caspcase-3 but also being inactivated by caspase-9 in the genisteininduced
apoptosis in gastric cancer BGC-823 cells.
Conclusion
Genistein induce the apoptosis of BGC-823 in dose- and timedependent manner. This apoptosis may be mediated by downregulating the apoptosis-associated Bcl-2 gene and up-regulating the expression of apoptosis-associated caspase-3 gene. As genistein was effective in vitro in promotion of apoptosis of gastric cancer cell by caspase-3 and Bcl-2 related apoptotic pathway, it might be a potential therapeutic agent against gastric cancer.
References
- Ilson DH. Angiogenesis in gastric cancer: hitting the target? Lancet. 2014; 383: 4-6.
- Thrumurthy SG, Chaudry MA, Hochhauser D, Mughal M. The diagnosis and management of gastric cancer. BMJ. 2013; 347: f6367.
- Rahman R, Asombang AW, Ibdah JA. Characteristics of gastric cancer in Asia. World J Gastroenterol. 2014; 20: 4483-4490.
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010; 127: 2893-2917.
- Cragg GM, Newman DJ, Yang SS. Natural product extracts of plant and marine origin having antileukemia potential. The NCI experience. J Nat Prod. 2006; 69: 488-498.
- Chevalier A, Alam MP, Khdour OM, Schmierer M, Arce PM, Cripe CD, et al. Optimization of pyrimidinol antioxidants as mitochondrial protective agents: ATP production and metabolic stability. Bioorg Med Chem. 2016; 24: 5206-5220.
- Koca SS, Dağlı AF, Yolbaş S, Gözel N, Işık A. Genistein protects dermal fibrosis in bleomycin-induced experimental scleroderma. Eur J Rheumatol. 2015; 2: 99-102.
- Kavoosi F, Dastjerdi MN, Valiani A, Esfandiari E, Sanaei M, Hakemi MG. Genistein potentiates the effect of 17-beta estradiol on human hepatocellular carcinoma cell line. Adv Biomed Res. 2016; 5: 133.
- Aichinger G, Beisl J, Marko D. Genistein and delphinidin antagonize the genotoxic effects of the mycotoxin alternariol in human colon carcinoma cells. Mol Nutr Food Res. 2016.
- Fiocchetti M, Cipolletti M, Leone S, Ascenzi P, Marino M. Neuroglobin overexpression induced by the 17β-Estradiol-Estrogen receptor-α Pathway reduces the sensitivity of MCF-7 Breast cancer cell to paclitaxel. IUBMB Life. 2016; 68: 645-651.
- Banerjee S, Li Y, Wang Z, Sarkar FH. Multi-targeted therapy of cancer by genistein. Cancer lett. 2008; 269: 226-242.
- Sarkar FH, Li Y. Soy isoflavones and cancer prevention. Cancer Invest. 2003; 21: 744-757.
- Bielecki A, Roberts J, Mehta R, Raju J. Estrogen receptor-beta mediates the inhibition of DLD-1 human colon adenocarcinoma cells by soy isoflavones. Nutr cancer. 2011; 63: 139-150.
- Han H, Zhong C, Zhang X, Liu R, Pan M, Tan L, et al. Genistein induces growth inhibition and G2/M arrest in nasopharyngeal carcinoma cells. Nutr cancer. 2010; 62: 641-647.
- Rahal OM, Simmen RC. PTEN and p53 cross-regulation induced by soy isoflavone genistein promotes mammary epithelial cell cycle arrest and lobuloalveolar differentiation. Carcinogenesis. 2010; 31: 1491-1500.
- Ullah MF, Ahmad A, Zubair H, Khan HY, Wang Z, Sarkar FH, et al. Soy isoflavone genistein induces cell death in breast cancer cells through mobilization of endogenous copper ions and generation of reactive oxygen species. Mol Nutr Food Res. 2011; 55: 553-559.
- Zhou HB, Chen JJ, Wang WX, Cai JT, Du Q. Apoptosis of human primary gastric carcinoma cells induced by genistein. World J Gastroenterol. 2004; 10: 1822-1825.
- Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006; 25: 4798-4811.
- Stennicke HR, Salvesen GS. Properties of the caspases. Biochim Biophys Acta. 1998; 1387: 17-31.
- Dang YM, Huang G, Chen YR, Xie XD. Sulforaphane inhibits the proliferation of the BIU87 bladder cancer cell line via IGFBP-3 elevation. APJCP. 2014; 15: 1517-1520.
- van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998; 31: 1-9.
- Shore GC, Nguyen M. Bcl-2 proteins and apoptosis: choose your partner. Cell. 2008; 135: 1004-1006.
- Means JC, Venkatesan A, Gerdes B, Fan JY, Bjes ES, Price JL. Drosophila spaghetti and double time link the circadian clock and light to caspases, apoptosis and tauopathy. PLoS Genet. 2015; 11:e1005171.
- Jeong SY, Seol DW. The role of mitochondria in apoptosis. BMB Rep. 2008; 41: 11-22.
- Abdel Shakor AB, Atia M, Alshehri AS, Sobota A, Kwiatkowska K. Ceramide generation during curcumin-induced apoptosis is controlled by crosstalk among Bcl-2, Bcl-xL, caspases and glutathione. Cell Signal. 2015; 27: 2220-2230.
- Genovese MI, Lopes Barbosa AC, Pinto MD, Lajolo FM. Commercial soy protein ingredients as isoflavone sources for functional foods. Plant Foods Hum Nutr. 2007; 62: 53-58.
- Banerjee S, Li YW, Wang ZW, Sarkar FH. Multi-targeted therapy of cancer by genistein. Cancer lett. 2008; 269: 226-242.
- Whyte L, Huang YY, Torres K, Mehta RG. Molecular mechanisms of resveratrol action in lung cancer cells using dual protein and microarray analyses. Cancer Res. 2007; 67: 12007-12017.
- Nahum A, Zeller L, Danilenko M, Prall OW, Watts CK, Sutherland RL, et al. Lycopene inhibition of IGF-induced cancer cell growth depends on the level of cyclin D1. Eur J Nutr. 2006; 45: 275-282.
- Zhang Y, Li Q, Chen H. DNA methylation and histone modifications of Wnt genes by genistein during colon cancer development. Carcinogenesis. 2013; 34: 1756-1763.
- Wang Z, Li Y, Ahmad A, Banerjee S, Azmi AS, Kong D, et al. Down-regulation of Notch-1 is associated with Akt and FoxM1 in inducing cell growth inhibition and apoptosis in prostate cancer cells. J cellular biochemistry. 2011; 112: 78-88.
- Chu SH, Lim JW, Kim DG, Lee ES, Kim KH, Kim H. Down-regulation of Bcl-2 is mediated by NF-kappaB activation in Helicobacter pylori-induced apoptosis of gastric epithelial cells. Scand J Gastroenterol. 2011; 46: 148-155.
- Broz P. Immunology: Caspase target drives pyroptosis. Nature. 2015; 526: 642-643.
- Liang S, Sun K, Wang Y, Dong S, Wang C, Liu L. Role of Cyt-C/caspases-9,3, Bax/Bcl-2 and the FAS death receptor pathway in apoptosis induced by zinc oxide nanoparticles in human aortic endothelial cells and the protective effect by alpha-lipoic acid. Chem Biol Interact. 2015; 258: 40-51.
- Hu J, Xu M, Dai Y, Ding X, Xiao C, Ji H, et al. Exploration of Bcl-2 family and caspases-dependent apoptotic signaling pathway in Zearalenone-treated mouse endometrial stromal cells. Biochem Biophys Res Commun. 2016; 476: 553-559.
- Huang YC, Kuo CL, Lu KW, Lin JJ, Yang JL, Wu RS, et al. 18α-Glycyrrhetinic Acid Induces Apoptosis of HL-60 Human Leukemia Cells through Caspases- and Mitochondria-Dependent Signaling Pathways. Molecules. 2016; 21: E872.
- Clavier A, Rincheval-Arnold A, Colin J, Mignotte B, Guénal I. Apoptosis in Drosophila: which role for mitochondria? Apoptosis. 2015; 21: 239-251.
- Vizetto-Duarte C, Custódio L, Gangadhar KN, Lago JH, Dias C, Matos AM, et al. Isololiolide, a carotenoid metabolite isolated from the brown alga Cystoseira tamariscifolia, is cytotoxic and able to induce apoptosis in hepatocarcinoma cells through caspase-3 activation, decreased Bcl-2 levels, increased p53 expression and PARP cleavage. Phytomedicine. 2016; 23: 550-557.