Research Article
Anatomic Trisegmentectomy: An Alternative Treatment for Huge or Multiple Hepatocellular Carcinoma of Right Liver
Jia Changku1*, Weng Jie2, Qin Qifan3, Chen Youke2 and Fu Yu2
1Department of Hepatobiliary Pancreatic Surgery, Nanjing Medical University, China
2Department of Hepatobiliary Pancreatic Surgery, The Affiliated Hospital of Hainan Medical University, China
3Department of General Surgery, Lin’gao County Hospital of Hainan Province, China
*Corresponding author: Jia Changku, Department of Hepatobiliary Pancreatic Surgery, Hangzhou First People’s Hospital, Nanjing Medical University, Hangzhou 310006, China
Published: 29 Nov, 2016
Cite this article as: Changku J, Jie W, Qifan Q, Youke C,
Yu F. Anatomic Trisegmentectomy:
An Alternative Treatment for Huge or
Multiple Hepatocellular Carcinoma of
Right Liver. Clin Oncol. 2016; 1: 1147.
Abstract
Background: The patients with huge (≥10 cm) or multiple Hepatocellular Carcinoma (HCC) in
right liver and insufficient volume of remnant left liver cannot be performed right hemihepatectomy
in that liver failure will occur post operation. We designed anatomic trisegmentectomy in right liver
to increase the percentage of future liver remnant volume (%FLRV), thus increasing the resectability
of huge or multiple HCC.
Methods: Thirteen patients were analysed by preoperative CT scan for liver and tumor volumetries.
If right hemihepatectomy was performed, %FLRV would be at the range of 29.6% - 37.5%. However,
if trisegmentectomy was done, %FLRV would increase by an average of 14.0%. So patients will
not undergo postoperative liver failure due to sufficient %FLRV. Therefore, we designed anatomic
trisegmentectomy, with retention of segment 5 or segment 8, to increase %FLRV and increase the
resectability for huge or multiple HCC.
Results: After trisegmentectomy, the inflow and outflow of remnant liver were maintained well.
Severe complications and mortality was not happened post operation. Of the 13 patients, 10 survived
up to now. Of the 10 living cases, postoperative lung metastasis was found in 2 and intra hepatic
recurrence was found in 1. These 3 patients survive with tumor after comprehensive therapies
including oral administration of Sorafenib.
Conclusion: Compared to right hemihepatectomy, anatomic trisegmentectomy in right liver
guarantees the maximum preservation of %FLRV to increase the resectability of huge or multiple
HCC, thus improving the overall resection rate.
Keywords: Anatomic segmentectomy; Hepatocellular carcinoma; Respectability; Liver volume
Introduction
Huge (≥10 cm) or multiple liver tumors often advance beyond any criteria of liver transplantation, and patients with huge or multiple liver tumors are also unable to benefit from radio frequency ablation. So hepatectomy is the only curative option for such patients [1-4]. However, complete resection of huge or multiple Hepatocellular Carcinoma (HCC) usually results in loss of major liver tissue in many such cases. So the radical resection cannot be performed if the percentage of future liver remnant volume (%FLRV) is too small or insufficient. For example, patients with huge or multifocal tumors in right liver and small volume of left liver cannot be performed right hemi hepatectomy in case of postoperation liver failure. Fortunately, in some cases, not all the 4 segments of right lobe (Couinaud segmentation) were involved by tumors though there are huge or multifocal tumors in right liver. %FLRV will be greatly increased if this uninvolved segment is preserved, thus decreasing the risk of postoperative liver failure and increasing the respectability of huge or multifocal HCC. In this study, we introduced anatomic trisegmentectomy including liver segmentectomy of 6, 7 and 8 and segmentectomy of 5, 6 and 7 to increase the respectability of huge or multiple HCC.
Material and Methods
Patients
Thirteen patients underwent anatomic trisegmentectomy from Feb 2012 to Jul 2015 in this study.
Of these 13 cases, 6 underwent 5, 6 and 7 segmentectomy and 7 underwent 6, 7, 8 segmentectomy.
All of them were male and their mean age was 58 years (range: 43-67 years). Laboratory examination showed that all the patients were positive of HBsAg. Ultrasound B and
CT scan showed that cirrhosis existed to varying degrees in all of the
livers. All patients had tumors in right liver with multiple lesions in
5 patients and huge lesion in 8 (Table 1 and 2). Maximal diameter of
the tumor ≥10 cm was huge HCC. Preoperative imaging showed that
maximal diameter of the tumor was 13.5cm. Two or three lesions of
tumor were referred to as multifocal tumors. Laboratory examination
showed that all patients had elevated serum α-fetoprotein (AFP).
Extra hepatic metastasis was ruled out by abdominal Ultrasound B,
chest CT and whole body bone scan prior surgery. Ethics approval:
The study was reviewed and approved by the Medical Ethics Board
of Hangzhou First People’s Hospital, Nangjing Medical University.
Informed consent: All study participants, or their legal guardian,
provided informed written consent prior to study enrollment.
Preoperative assessment
Preoperative assessments including hepatic function, hepatic
functional reserve and hepatic imaging were examined. The test
of indocyanine green retention at 15 min (ICG-R15) was used
to evaluate hepatic functional reserve (Table 1 and 2). Manual 3D
reconstructions of the liver by contrast-enhanced CT were made
preoperatively. Total liver, left liver and segments of right liver,
as well as the tumors were manually outlined and their volumes
were calculated as reported [5,6]. %FLRV was calculated using
the formula: %FLRV = (remnant liver volume) × 100/(total liver
volume - tumor volume) [7]. Liver volumetry showed that if right
hemihepatectomy was performed, %FLRV would be at the range of
29.6%-37.5% in this study (Table 1 and 2). The risk of postoperative
liver failure would be high due to insufficient %FLRV. However,
if 5,6,7 segmentectomies were performed in 6 patients, %FLRV
would increase by an average of 14.5%. If 6,7,8 segmentectomies
were performed in 7 patients, %FLRV would increase by an average of 13.6%. Compared to right hemihepatectomy, %FLRV would
increase by an average of 14.0% if trisegmentectomies including
5,6,7 segmentectomies and 6,7,8 segmentectomies were performed
(Table 1 and 2). Trisegmentectomies decrease the risk of liver failure
post operation due to increased %FLRV. So we designed anatomic
trisegmentectomy, with retention of segment 5 or 8 respectively, to
increase the resectability of huge or multiple HCC.
Surgical procedures
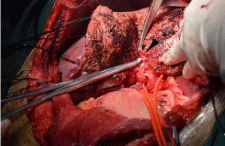
Liver resection line was determined by selective hepatic inflow
occlusion. After cholecystectomy, the right hemihepatic Glissonean
pedicle and the segment 6,7 Glissonean pedicle were sequentially
divided. Demarcation between segment 6,7 and segment 5,8 could be
determined by ligation of the segment 6,7 Glissonean pedicle (Figure
1). Then the right hemihepatic Glissonean pedicle was occluded. So the interface between segment 5,8 and segment 4 can be demarcated
(Figure 2). After demarcation, the right hemihepatic Glissonean
pedicle was unoccluded. Then, for 5,6,7 segmentectomy, the area
of segment 5 could be demarcated by dissection and occlusion of
the branch pedicles of segment 5 during parenchymal transection
(Figure 3-6). Finally, a “┕┓” shape- like broken resection line
could be demarcated upon the diaphragmatic surface of the liver.
For 6,7,8 segmentectomy, the area of segment 8 was determined by
the technique of intraoperative ultrasound as reported [5]. Finally, a
“┏┛” shape- like broken resection line could be demarcated upon
the diaphragmatic surface of the liver. Liver resection was completed
along the broken resection line. Then the tumor free segment 5 or 8
would be reserved during trisegmentectomy in right liver. If needed,
only right hemihepatic inflow occlusion was used to reduce blood loss during liver resection. Parenchymal transection was performed using
ultrasonic scalpel and cavitron ultrasonic surgical aspirator (CUSA).
Postoperative management
Postoperative follow-up and postoperative check-up were
performed on time. Tests of liver function, assay of serum AFP and
imaging studies were examed at regular intervals. Because huge or
multifocal tumors are risk factors for recurrence, so all of the patients
in this study were given 3 times therapy of transcatheter arterial
chemoembolization (TACE) post operation in order to prevent
recurrence in the remnant liver. TACE was given at intervals of 30 d
in the first 3 months post operation. Sorafenib, the molecular targeted
anti-tumor drug for HCC was given for those metastatic or recurrent
patients.
Table 1
Table 1
Clinical features and postoperative outcomes of patients underwent 5, 6, 7 segmentectomy.
DFS: disease-free survival.
Table 2
Table 2
Clinical features and postoperative outcomes of patients underwent 6, 7, 8 segmentectomy.
DFS: disease-free survival.
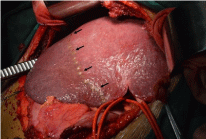
Figure 1
Figure 1
Right hemihepatic Glissonean pedicle and segment 6,7 Glissonean
pedicle of case 5 in 5,6,7 segmentectomy group were sequentially divided.
Demarcation between segments 6,7 and segment 5,8 was determined by
ligation of the segment 6, 7 Glissonean pedicle. Arrow: interface between
segments 6,7 and segment 5,8.
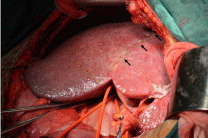
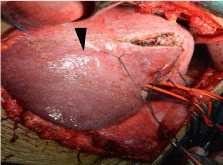
Figure 2
Figure 2
Right hemihepatic Glissonean pedicle of case 5 in 5,6,7
segmentectomy group was occluded. So the interface between right and left
liver was demarcated (arrow).
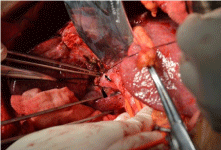
Figure 3
Figure 3
A branch pedicle of segment 5 of case 5 in 5,6,7 segmentectomy
group was dissected and occluded. Arrow: A branch pedicle of segment 5.
Triangle arrow: Glissonean pedicle of segment 6,7.
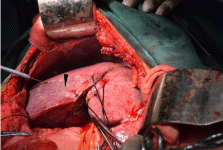
Figure 4
Figure 4
After occlusion of one branch pedicle of segment 5, a ischemic
area of segment 5 (triangle arrow) was marked upon the diaphragmatic
surface of the liver.
Figure 5
Figure 5
Another branch pedicle of segment 5 (triangle arrow) of case 5 in
5,6,7 segmentectomy group was dissected.
Figure 6
Figure 6
After occlusion of the branch pedicle of segment 5, total ischemic
area of segment 5 (triangle arrow) was marked upon the diaphragmatic
surface of the liver. Finally, a “┕┓” shape- like broken resection line was
marked upon the diaphragmatic surface of the liver.
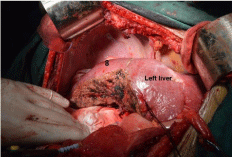
Figure 7
Figure 7
After hepatectomy, the inflow and outflow of segment 5 of case
5 in 5,6,7 segmentectomy group were maintained. Segment 8 and left liver
was indicated.
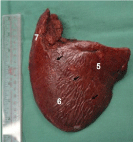
Figure 8
Figure 8
Gross specimen showed that tumors were completely resected.
Segments 5,6 and 7 were indicated. Arrow: interface between segment 6 and
segment 5, which was demarcated in operation.
Results
Anatomic trisegmentectomy in right liver was completed
uneventfully for all of the patients, with a mean operative time of
285min (210-470 min) and a mean blood loss of 720ml (400–1800
ml). After trisegmentectomy, the inflow and outflow of remnant liver
were maintained well (Figure 7). Gross specimens showed that tumors
were totally removed (Figure 8) and hepatocellular carcinomas
were verified by postoperative pathology. There were no perioperative
mortality and server postoperative complications. AFP level of
all patients reduced to the normal range within two months post
operation.
Of the 13 patients, 10 survived up to now, with the longest surviving
time of 4 years. One patient in the group of 6,7,8 segmentectomy died
383 d postoperatively due to obstructive supportive cholangitis of
unknown causes. Another one in this group died from intrahepatic
multiple recurrence and liver failure at 802 d post operation. One
patient underwent segmentectomy 5,6,7 died at 310 days due to the
multiple intrahepatic metastasis and liver failure. Of the 10 living
cases, postoperative lung metastasis was found in 2 and intrahepatic
recurrence was found in 1. These 3 patients survive with tumor after
comprehensive therapies including oral administration of Sorafenib.
Qualities of life of these patients are well. Postoperative outcome are
summarized in Table 1 and 2.
Discussion
It has been a major topic for hepatobiliary surgery to increase
the safety and resection rate for HCC by increasing liver remnant
volume [8-12]. The patients with huge or multiple HCC in right liver
and insufficient volume of remnant left liver cannot be performed
right hemihepatectomy in that liver failure will occur post operation.
For all the patients in this study, liver volumetry showed that if
right hemihepatectomy was performed, %FLRV would be at the
range of 29.6% - 37.5%. These patients cannot be performed right
hemihepatectomy due to liver cirrhosis and insufficient %FLRV.
However, compared to right hemihepatectomy, %FLRV would
increase by an average of 14.0% if trisegmentectomies including
5,6,7 segmentectomies and 6,7,8 segmentectomies were performed.
So these patients can be performed right hemihepatectomy due to
sufficient %FLRV. Therefore these patients obtained the opportunity
Figure 8: Gross specimen showed that tumors were completely resected.
Segments 5,6 and 7 were indicated. Arrow: interface between segment 6 and
segment 5, which was demarcated in operation.
to perform the curative operation because of sufficient remnant
functional liver. And because of anatomic resection, it makes
the maximum preservation of functional liver tissue and
complete tumor excision as well as tumor-free margins [13,14].
In this study, hepatectomies were uneventfully completed
with a mean operative time of 285min (210-470 min) and a mean
blood loss of 720ml (400 – 1800ml). There were no perioperative
mortality and server postoperative complications like postoperative
abdominal bleeding and bile leakage. The blood loss in our study
(mean: 720 mL) equals to that reported in many literatures [15,16].
For treatment effects, serum AFP reduced to the normal range
within 2 months post operation in all patient, which indicate that
anatomic trisegmentectomy in right lobe can achieve the goal of
complete tumor excision.
All of the 13 patients have survived more than 6 months
postoperation. Ten of them survived up to now, with
the longest surviving time of 4 years. Although postoperative lung
metastasis was found in 2 and intrahepatic recurrence was found
in 1 among the 10 living cases, these 3 patients survive with tumor
after comprehensive therapies including oral administration of
Sorafenib. Overall, patients in this study achieved satisfied shortterm
survival and good life quality postoperation, which indicated
that trisegmentectomy had a good therapeutic efficacy for huge and
multifocal tumors.
Techonlogically, the approach of Glissonean pedicle dissection
benefits anatomic trisegmentectomy of right liver. There is a
safe plane between Glissonean pedicle and the liver parenchyma
along which dissection of Glissonean pedicle is simple, convenient,
practical and time-saving with reduced damage of vasculars [17]. Then
two steps of Glissonean pedicle occlusion were used to determine the
resection line. For 5,6,7 segmentectomy, after demarcation between
the segment 6,7 and segment 5, 8 as well as right liver and left liver, the
area of segment 5 could be demarcated by dissection and occlusion of
the branch pedicles of segment 5 during parenchymal transection
(Figure 3-6). As for 6,7,8 segmentectomy, parenchyma transection
between segment 5 and segment 4 will be performed if the branch
pedicles of segment 8 be dissected and isolated because of deep-seated
of the pedicle of segment 8. And the risk of damage to the branch
pedicles of segment 5 will be high during dissection and parenchyma
transection. So it was unnecessary to compulsorily isolate the branch
pedicles of segment 8. For 6,7,8 segmentectomy, the area of segment
8 was determined by the technique of intraoperative ultrasound B
with a transverse marked line upon the diaphragmatic surface of the
liver between segments 8 and 5. Finally, a “┏┛” shape- like broken
resection line was demarcated.
In addition, two steps of Glissonean pedicle isolation guarantees
selective occlusion of right hemihepatic inflow afterward. If needed,
only right hemihepatic inflow occlusion was used to reduce blood loss
during trisegmentectomy in this study. This technique enables blood
inflow to left liver and avoids splanchnic stasis during the whole
resection process [17-19]. Thus, there was no total hepatic ischemiareperfusion
injury and hemodynamic instability. It particularly
benefits patients with liver cirrhosis [17,18].
Acknowledgement
This research was supported by Grant of the Application Research and Demonstration & Promotion of Hainan Province, No. ZDXM2014074, Program of Social Development and Scientific and Technological Projects of Hainan Province, No. SF201422.
References
- Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, et al. Sustained complete response and complications rates after radiofrequency ablation of very early HCC in cirrhosis: Is resection still the treatment of choice?. Hepatology. 2008; 47: 82-89.
- Andreou A, Vauthey JN, Cherqui D, Zimmitti G, Ribero D, Truty MJ, et al. Improved long-term survival after major resection for hepatocellular carcinoma: a multicenter analysis based on a new definition of major hepatectomy. J Gastrointest Surg. 2013; 17: 66-77.
- Dittmar Y, Altendorf-Hofmann A, Schüle S, Ardelt M, Dirsch O, Runnebaum IB, et al. Liver resection in selected patients with metastatic breast cancer: a single centre analysis and review of literature. J Cancer Res Clin Oncol. 2013; 139: 1317-1325.
- Zhou L, Rui JA, Wang SB, Chen SG, Qu Q. Risk factors of poor prognosis and portal vein tumor thrombosis after curative resection of solitary hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2013; 12: 68-73.
- Jia C, Weng J, Chen Y, Fu Y. Anatomic resection of liver segments 6-8 for hepatocellular carcinoma. World J Gastroenterol. 2014; 20: 4433-4439.
- de Graaf W, van Lienden KP, Dinant S, Roelofs JJ, Busch OR, Gouma DJ, et al. Assessment of future remnant liver function using hepatobiliary scintigraphy in patients undergoing major liver resection. J Gastrointest Surg. 2010; 14: 369-378.
- Okabe H, Beppu T, Chikamoto A, Hayashi H, Yoshida M, Masuda T, et al. Remnant liver volume-based predictors of postoperative liver dysfunction after hepatectomy: analysis of 625 consecutive patients from a single institution. Int J Clin Oncol. 2014; 19: 614-621.
- Chun YS, Ribero D, Abdalla EK, Madoff DC, Mortenson MM, Wei SH, et al. Comparison of two methods of future liver remnant volume measurement. J Gastrointest Surg. 2008; 12: 123-128.
- Di Domenico S, Santori G, Balbis E, Traverso N, Gentile R, Bocca B, et al. Biochemical and morphologic effects after extended liver resection in rats: preliminary results. Transplant Proc. 2010; 42: 1061-1065.
- Ratti F, Schadde E, Masetti M, Massani M, Zanello M, Serenari M, et al. Strategies to Increase the Resectability of Patients with Colorectal Liver Metastases: A Multi-center Case-Match Analysis of ALPPS and Conventional Two-Stage Hepatectomy. Ann Surg Oncol. 2015; 22: 1933-1942.
- Eshkenazy R, Dreznik Y, Lahat E, Zakai BB, Zendel A, Ariche A. Small for size liver remnant following resection: prevention and management. Hepatobiliary Surg Nutr. 2014; 3: 303-312.
- Leung U, Simpson AL, Araujo RL, Gönen M, McAuliffe C, Miga MI, et al. Remnant growth rate after portal vein embolization is a good early predictor of post-hepatectomy liver failure. J Am Coll Surg. 2014; 219: 620-630.
- Chen J, Huang K, Wu J, Zhu H, Shi Y, Wang Y, et al. Survival after anatomic resection versus nonanatomic resection for hepatocellular carcinoma: a meta-analysis. Dig Dis Sci. 2011; 56: 1626-1633.
- Hasegawa K, Kokudo N, Imamura H, Matsuyama Y, Aoki T, Minagawa M, et al. Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg. 2005; 242: 252–259.
- Liau KH, Ruo L, Shia J, Padela A, Gonen M, Jarnagin WR, et al. Outcome of partial hepatectomy for large (>10 cm) hepatocellular carcinoma. Cancer. 2005; 104: 1948-1955.
- Chen X, Wu Z, Qiu F.Hepatectomy for huge primary liver cancer: report of 171 patients. Zhonghua Wai Ke Za Zhi. 2000; 38: 6-9.
- Dello SA, Reisinger KW, van Dam RM, Bemelmans MH, van Kuppevelt TH, van den Broek MA, et al. Total intermittent Pringle maneuver during liver resection can induce intestinal epithelial cell damage and endotoxemia. PLoS One. 2012; 7: e30539.
- Giordano M, Lopez-Ben S, Codina-Barreras A, Pardina B, Falgueras L, Torres-Bahi S, et al. Extra-Glissonian approach in liver resection. HPB (Oxford). 2010; 12: 94-100.
- Yanaga K, Matsumata T, Nishizaki T, Shimada M, Sugimachi K. Alternate hemihepatic vascular control technique for hepatic resection. Am J Surg. 1993; 165: 365-366.