Case Report
Emerging Treatment Options for Adolescents and Young Adults with Relapsed or Refractory Lymphoma
Nmazuo W. Ozuah1 and Ann S. LaCasce2*
1Department of Pediatric Oncology, Dana Farber Cancer Institute, USA
2Department of Medical Oncology, Dana Farber Cancer Institute, USA
*Corresponding author: Ann S. LaCasce, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Avenue Boston, MA 02215, USA
Published: 23 Nov, 2016
Cite this article as: Ozuah NW, LaCasce AS. Emerging
Treatment Options for Adolescents
and Young Adults with Relapsed or
Refractory Lymphoma. Clin Oncol.
2016; 1: 1141.
Abstract
Lymphomas are amongst the most common cancers in Adolescents and Young Adults (AYA). Despite favorable outcomes with upfront therapy, survival in patients with relapsed or refractory disease remains poor. Promising therapeutic agents are emerging, including monoclonal antibodies, antibody drug conjugates, immune checkpoint inhibitors, cytotoxic T-cell therapies, small molecule pathway inhibitors and epigenetic modulators. Clinical trials of novel agents have predominantly enrolled older adult patients. Furthermore, research relating to the biology of lymphomas has focused predominately on younger children and older adults. Future investigation should concentrate on the biology of lymphomas in AYAs and improving their participation in clinical trials.
Introduction
Lymphoid malignancies are among the most common cancers in Adolescents and Young
Adults (AYA), defined as 15-39 years [1,2]. Classical Hodgkin Lymphoma (HL), which is the most
common hematologi malignancy in AYAs, accounts for up to 12% of cancers seen in patients aged
15-29 years [2]. Despite excellent outcomes with upfront therapy, a small proportion of patients will
have primary refractory disease or experience relapse [3]. Salvage chemotherapy followed by highdose
chemotherapy with autologous stem cell transplant (ASCT) remains the standard of care but
induces long-term remissions in only approximately 50% of patients [4].
Non-Hodgkin Lymphoma (NHL) represents a heterogeneous group of lymphoid malignancies
of B, T and NK–cell origin often separated into aggressive e.g. diffuse large B-cell lymphoma
(DLBCL), Burkitt lymphoma (BL), and anaplastic large cell lymphoma (ALCL); and indolent
forms [5]. DLBCL predominates in AYA patients, comprising about 60% of NHL cases [2,6]. Gene
expression profiling has revealed DLBCL can be classified into two major variants. The germinal
center B-Cell (GCB) subtype which is more common in children and AYAs; and the less favorable
activated B-cell (ABC) DLBCL mostly seen in older adults [7]. Similarly, salvage chemotherapy
followed by ASCT is also the standard treatment for relapsed/refractory DLBCL [8]. As in HL,
the inability to achieve a metabolic complete remission by functional imaging is associated with
worse post-transplant outcomes irrespective of type of salvage regimen used [9-12] and patients
who are transplant ineligible or have relapsed after transplant have very limited options. Options
for patients with relapsed, indolent NHLs include novel drugs, such as lenalidomide and idelalisib;
and as well, stem cell transplantation is considered for those who are resistant to rituximab and
alkylating agents [13]. In addition, there is no consensus regarding optimal treatment for relapsed/
refractory peripheral T-cell lymphoma (PTCL), which continues to be associated with a very poor
prognosis [14]. These highlight the need to develop effective treatment strategies for relapsed and
refractory lymphoma.
Here we review some of the emerging therapeutic options in patients who have failed front-line
therapy, focusing on HL and DLBCL which are the most common lymphomas in AYAs.
AYA Lymphoma and Disease Biology
In general, improvement in overall survival in AYAs has lagged behind that of younger and older
patients with cancers. While the reasons are not well understood, they likely include differences
in adherence, care delivery and disease biology [15-17]. Whereas outcomes in younger children
have been advanced through participation in cooperative group clinical trials, participation by
adolescents in either pediatric or adult clinical trials remains low [18,19].
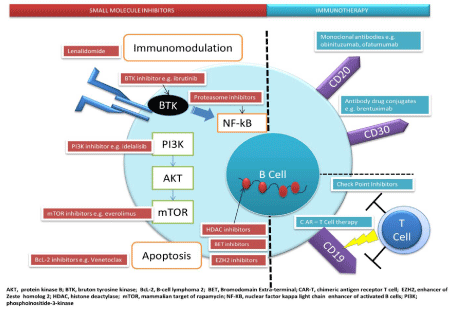
In recent years our understanding of lymphoma biology and the
pathways that drive oncogenesis has evolved, leading to opportunities
to explore novel targeted therapies. Some of these pathways and their
targets are illustrated in Figure 1. Unfortunately, studying the biology
of lymphomas in AYAs has not kept pace with our understanding of
the disease in children and older adults. Hence, strategies for treating
this population have not been clearly defined [2]. These novel agents
have been tested in clinical trials, typically in older adult populations.
We recognize that the young adults are underrepresented in many
trials; particularly the B-NHL studies and differences in biology across
age groups, likely inform treatment responses. Nonetheless, we feel
the results of these studies may warrant extrapolation to adolescent
and young adult populations.
Figure 1
Immunotherapy
Monoclonal antibodies targeting cell surface antigens
The emergence of the anti-CD20 monoclonal antibody (mAb)
rituximab significantly improved outcomes in the first-line treatment
of all CD20+ NHLs, particularly DLBCL and follicular lymphoma
(FL) [20,21]. Targeting CD20 should have similar efficacy in B-cell
lymphomas in AYAs. While multiple studies of adult B-NHL
demonstrated efficacy of rituximab, the experience in pediatrics
lagged behind, perhaps due to historically good outcomes in children
and adolescents. The Intergroup Trial for children and adolescents
with B-NHL (ANHL1131), a randomized phase 3 study, compared
the current chemotherapy backbone (LMB96) with LMB96
chemotherapy plus rituximab in patients with high risk B-NHL. The
results of this study have not been published, but interim analysis
showed superior 1-year EFS in the rituximab arm (94.2% vs. 81.5%
in the non-rituximab arm). Hence, the current recommendation
for treating newly diagnosed B-NHL in pediatrics is rituximab plus
chemotherapy.
Despite the successes with rituximab, the development of
rituximab-refractory disease led to the development of other anti-
CD20 mAbs which may have improved efficacy [22]. Ofatumumab
and obinutuzumab represent newer generation anti-CD20
mAbs which have shown clinical activity in relapsed/refractory
NHLs. Obinutuzumab (GA101) is the first humanized type II
glycoengineered anti-CD20 mAb. It has a low level of complementdependent
cytotoxicity and increased direct non-apoptotic cell
death, compared to rituximab based on in vitro and in vivo studies
[22]. Used as a single agent in both relapsed/refractory indolent and
aggressive B-NHLs, obinutuzumab resulted in objective response
rates (ORR) of 55% and 28% respectively, with a median progression
free survival (PFS) of 11.9 months in the indolent NHL cohort [23,24].
The median age of patients in that study was 71 years (range 22-85
years). Improved response rates of 93 to 96% were observed when
this agent was added to a chemotherapy backbone in patients with
relapsed/refractory FL [25]. The drug is currently being evaluated in
combination with other agents in ongoing clinical trials in both the
relapsed and upfront settings.
Of atumumab is a fully humanized second generation type I anti-
CD20 mAb. Preclinical data suggested it had improved complementdependent
cytotoxicity compared with rituximab [26] and superior
activity against both rituximab-sensitive and resistant cell lines
[27]. Coiffier et al. [28,29] first reported ORR of 44% in relapsed/
refractory Chronic Lymphocytic Leukemia (CLL) patients, which led
it to its regulatory approval for this indication. However it is yet to
demonstrate any clinical activity in relapsed/refractory DLBCL [30].
Monoclonal antibodies targeting other B-cell surface antigens
have also been developed, although these have not been approved for
use in the United States. One of such is epratuzumab, a humanized
mAb against CD22, which is expressed by most NHLs. Combined
with the radioisotope Yitrium90 in a phase 1 study of adults with
relapsed/refractory aggressive NHL, ORR of 53% was observed. This
included response rates of 50% in the DLBCL group, and responses in
all four evaluable patients in the transformed FL group [31]. MEDI-
551 an anti-CD19 monoclonal antibody also demonstrated single-
agent activity in a cohort that included both relapsed/refractory FL
and DLBCL patients (ORR 24%) [32].
Antibody-Drug conjugates
Antibody–drug conjugates (ADCs) consist of anti-cancer agents
covalently linked to monoclonal antibodies directed at antigens
which are differentially over expressed in tumor cells. Brentuximab
vedotin (SGN-35) is a CD30-directed antibody conjugated to the
anti-microtubule monomethyl auristatin E (MMAE). CD30, a transmembrane
glycoprotein is expressed on the Reed Stenberg cells in
HL and on ALCL cells [33]. Younes et al. [4] observed 75% ORR and
a 34% Complete Response (CR) in 102 heavily pre-treated patients
(median age 31 years) with relapsed/refractory HL. The median
duration of response (DOR) was 20.5 months in those who achieved a
CR. Following these results; several other studies have demonstrated
similar and durable responses in relapsed/refractory HL [34-36].
Brentuximab also showed remarkable activity in relapsed/refractory
ALCL (ORR 86%, CR 57%) [37].
The Food and Drug Administration (FDA) has approved
brentuximab for treatment of patients with refractory HL post-
ASCT, transplant ineligible HL patients after failure of at least two
prior multi-agent chemotherapy regimens, and relapsed/refractory
ALCL after failure of at least one prior multi-agent chemotherapy
regimen. Brentuximab has also been used successfully as a bridge
to transplant in refractory HL patients [34]. Combined with the
alkylator bendamustine in pre-ASCT patients with relapsed or
refractory HL, ORR of 93% with a CR of 76% was observed and
majority of these patients proceeded to ASCT (at 18 months, PFS of
75%) [38]. Although some activity was observed in CD30+ NHL [39],
its role in B-NHL has not been established. The drug is generally well
tolerated. The most common reported adverse events are neuropathy,
predominantly sensory, and hematologic toxicities [40].
Polatuzumab vedotin consists of an anti-CD79b monoclonal
antibody, and like brentuximab is conjugated to MMAE. A phase 1
study of single-agent polatuzumab vedotin in patients with relapsed/
refractory NHL showed ORR of 55% in 42 evaluable patients. In
addition, 7 out of 9 patients treated with both polatuzumab and
rituximab had an objective response [41]. Similar to brentuximab,
the most common adverse events have been neutropenia, anemia and
peripheral sensory neuropathy [41]. Phase 2 studies of polatuzumab
in combination with other agents - NCT02611323 and NCT02600897;
are currently active and enrolling patients. Denintuzumab mafodotin
(SGN-CD19A), an anti-CD19 antibody conjugated to monomethyl
auristatin F (MMAF), a second generation microtubule inhibitor, is
also being evaluated in phase 1/2 trials for relapsed/refractory NHL.
Immune check point inhibitors
Recent evidence has demonstrated a variety of tumors are able
to evade the host immune system through immune check point
pathways such as cytotoxic T-lymphocyte associated protein 4
(CTLA-4) and programmed-death 1 (PD-1) [42]. Initial preclinical
and clinical benefits of check point blockade were first demonstrated
in melanoma [43,44] Subsequent data in HL demonstrating PD-1
related immune evasion by Reed-Sternberg cells have led to early
phase studies of PD-1 inhibitors in this disease [45]. Ansell et al. [46]
reported ORR of 87%, PFS of 86% at 24 weeks, following single agent
PD-1 blockade with nivolumab in 23 heavily pre-treated relapsed/
refractory HL patients. Also, Younes et al. [47] recently published
results of their ongoing single-arm phase 2 study of nivolumab in
adult patients with recurrent HL who had failed both ASCT and
brentuximab. They reported ORR of 66% among their cohort of 80
patients (age range 28-48 years, median 37 years), which was identical
to findings observed by Armand et al. [48] in the phase 1 study of
pembrolizumab in relapsed/refractory HL patients after brentuximab
failure (ORR 66%, CR 16%, PFS 69% at 24 weeks and 46% at 52
weeks). These findings recently led to the accelerated FDA approval
of nivolumab for patients with recurrent HL.
PD-1 appears to be important in the biology of subsets of NHL,
including Epstein Barr Virus (EBV) - associated lymphomas. In
a study of nivolumab, ORR of 36% and 40% were also reported in
DLBCL and FL respectively [49]. An earlier phase 1 study of the
CTLA-4 inhibitor, Ipilimumab had not demonstrated any significant
activity in NHL [50]. In patients with relapsed FL, PD-1 inhibitor
pidilizumab in combination with rituximab resulted in ORR of
66% (CR 52%), [51] supporting a role for check point blockade in
relapsed/refractory NHL. In parallel with on-going investigation into
the biologic importance of these pathways in lymphoma, clinical
trials combining different PD-1 inhibitors with other agents are in
progress.
Table 1
Table 2
Table 2
Novel small molecule inhibitors in clinical investigation for relapsed/refractory lymphomas.
Cytotoxic T-Cell Therapy
Chimeric antigen receptor (CAR) T cells
Chimeric antigen receptor (CAR) -T cells are genetically
engineered immune effect or cells targeted to tumor cells. Their
basic construct includes an extracellular tumor antigen recognition
domain, fused to an intracellular T-cell activating signaling domain
which most commonly includes the trans membrane adaptor
signaling protein CD3ζ [52]. Initial trials of CD19-targeted CAR -T
cells focused on CLL with the assumption that this disease as well
as indolent lymphomas like FL would allow time for mobilization
and engineering of patient T cells without concerns for treatment
delays [53-56]. However it was the remarkable efficacy in B-Acute
lymphoblastic leukemia (ALL) that asserted the role of CAR- T cells
in the treatment of hematological malignancies. Although preclinical
studies were promising no significant clinical responses were observed
with first generation CAR until the advent of second generation CAR
designs which incorporate intracellular signaling domains such as
CD28 and 4-1BB co-stimulatory domains, in addition to the CD3ζ
activation domain [54]. Today, third generation CARs with two costimulatory
domains are available.
Complete remission rates as high as 90% have been reported
in relapsed/refractory ALL patients treated with the CD19 CAR
-T cell therapy [57,58]. Responses in relapsed/refractory mature
B-cell lymphomas have also shown great promise. Kochenderfer et
al. [59] reported durable clinical responses of 9-22 months in 12 of
13 evaluable patients (including 8 patients with a CR) with chemorefractory
DLBCL and indolent NHL who received CD19 CAR -T
cells. The median age in the study was 56 years (range 30-68 years).
Other trials have also reported favorable responses [60,61]. Major
adverse events observed in these studies have been fever, hypotension,
delirium, and neurologic toxicities, but these have been mostly
reversible [62]. Other CAR -T cells being evaluated include CD22
CAR-T (NCT02315612) and CD30 CAR-T for relapsed/refractory
HL and ALCL (NCT02274584). A trial of sequential therapy CD19
and CD20 CAR-T for DLBCL (NCT02737085) is also ongoing.
Investigators are also evaluating combination of CAR-T cells with
other types of immunotherapy.
Bispecific T-cell engagers
Bispecific T-cell engagers (BiTE) are fusion proteins consisting of
two antibodies; one component which binds a surface target antigen
on cancer cells and the other to CD3 on T cells. Blinatumomab is
a CD19/CD3 BiTE that cross-links CD19 on tumor cells with CD3
on patient's cytotoxic T cells. Initial clinical efficacy data were
generated in a phase 1 trial of patients with indolent NHL, where
partial or complete tumor regression was observed [63]. Topp et
al. [64-67] then demonstrated that blinatumomab induced durable
minimal residual disease (MRD) negative status in adult patients
with ALL who had MRD persistence or relapse after induction and
consolidation therapy. Subsequently, the same group conducted a
phase 2 trial evaluating clinical efficacy in 36 patients with relapsed/
refractory ALL. They reported that single-agent blinatumomab
in relapsed /refractory ALL had a CR rate of 69% with median OS
of 9.8 months. Given its impressive activity in this group, the drug
was recently approved for use in relapsed/refractory Philadelphia
chromosome negative B-ALL.
Goebeler et al. [68] recently published their phase I study of
blinatumomab in patients with relapsed/refractory NHL, showing
an overall response of 69% across NHL subtypes, including ORR
55% in DLBCL. The most common adverse events reported were
fever and fatigue which were observed in 86% and 55% of patients
respectively. A Phase 1 study of blinatumomab in combination with
lenalidomide (NCT02568553) for patients with relapsed NHL is
currently underway.
Table 3
Table 3
Novel small molecule inhibitors in clinical investigation for relapsed/refractory lymphomas (continued).
Small Molecule Inhibitors
Immune modulators: Lenalidomide
Lenalidomide is an immunomodulatory agent with antitumor
activity particularly in B-cell malignancies. Recent studies have
elucidated its mechanism of action via binding to the ubiquitin E3
ligase cereblon, leading to degradation of the transcription factors
IKZF1 and IKZF3 [69]. In a phase 2 trial that enrolled 217 patients
(median age, 66 years) with relapsed/refractory NHL, lenalidomide
demonstrated clinical activity with ORR of 35%. Fifty percent of
patients enrolled in that study had DLBCL [70]. In a subsequent
study, Hernandez et al. [71,72] observed a differential response
to lenalidomide - 52.9% in the ABC subtype versus 8.7% in GCBDLBCL.
Since its activity may be restricted to non-GCB DLBCL and
not GCB DLBCL which predominates in AYAs, this agent may not
have significant applicability in this population. Activity in HL has
been modest [73]. Combination therapy with other novel agents is
being evaluated.
Targeting the PI3K/AKT/mTOR pathway
The mammalian target of rapamycin (mTOR), a downstream
effect or of the phosphatidylinositol 3-kinase (PI3K)/Akt (protein
kinase B) signaling pathway, mediates cell survival and proliferation.
Constitutive activation of the PI3K/AKT pathway and mTOR kinase
signaling has been recognized as critical events in lymphomagenesis
[74]. In addition, mTOR plays an important role in anticancer drug
resistance [75]. A phase 2 trial of single agent mTOR inhibitor
everolimus in relapsed/refractory DLBCL resulted in ORR of 30%
[76]. Its efficacy and safety in combination with rituximab was also
investigated in 26 patients (median age 65 years) with DLBCL who
had failed or were ineligible to receive ASCT. The evaluable patients
in that study had ORR of 38% [77]. The North Central Cancer
Treatment Group (NCCTG) recently reported complete metabolic
remission of 96% in 24 patients who received a combination of
standard R-CHOP and everolimus as upfront therapy for DLBCL.
None of the patients had relapsed after median follow up of 21.5
months. The most common grade 3-4 event was neutropenia (75%
of patients) [78]. Everolimus has also showed some activity in HL.
Johnston et al. [79] reported ORR of 47% in a cohort of patients with
relapsed HL.
Pre-clinical studies combining everolimus and the histone
deacetylase (HDAC) inhibitor panobinostat showed synergistic antiproliferative
activity [80]. Phase 1 study combining these two agents
in both relapsed/refractory HL and NHL showed that 33% of patients
achieved a clinical response including 3 CRs, with ORR 43% and 25%
in HL and NHL patients respectively. Disappointingly, none of the
DLBCL patients in that study obtained a measurable response [81].
Epigenetic Modulators: HDAC inhibitors
By modifying gene expression, histone deacetylase inhibitors
(HDACi see below) have been shown to be active in malignancies
including relapsed/refractory lymphomas [82]. Panobinostat
demonstrated single agent activity in a cohort of HL patients who had
failed ASCT (ORR 27%) [83]. Notably, a reduction in serum thymus
and activation-regulated chemokine (TARC) levels was observed
in patients who had disease control suggesting that response could
be measured by serial levels of this protein [83,84]. However other
HDACi have not shown much clinical benefit in HL [85,86].
Both Belinostat and romidepsin demonstrated durable clinical
responses in relapsed/refractory PTCL and are currently FDA
approved for this indication [87,88]. Although vorinostat showed
modest efficacy in indolent NHL (ORR 28%), [85] it had limited
activity in DLBCL (ORR 5.6%) [89]. Pre-clinical studies had suggested
the anti-tumor activity of this class of drugs could be potentiated in
combination with other agents [90,91], but unfortunately this has
been limited by hematologic toxicity, particularly thrombocytopenia
[92]. Younes et al. [93] recently reported response rates of 55% in
relapsed/refractory DLBCL with CUDC-907 a combined HDAC and
PI3K inhibitor. In that study, the combination was quite tolerable
with grade 3 or worse thrombocytopenia only reported in 20% of the
patients.
Epigenetic Modulators: EZH2 inhibitors
The histone methyltransferase EZH2 is the catalytic subunit
of the polycomb repressive complex 2 (PRC2) which is required
for epigenetic silencing of chromatin [94]. It is frequently mutated
in GCB-DLBCL and FL [95-97], and has become increasingly
recognized in different types of cancers as a potential drug target.
Building on pre-clinical studies reporting clinical activity in
EZH2 mutant NHL [98], there are currently three open phase 1/2
clinical trials evaluating EZH2 inhibitors in relapsed/refractory
lymphomas- CPI-1205 (NCT02395601), E7438 (NCT01897571) and
GSK2816126 (NCT02082977). Given GCB -DLBCL predominates
in AYA lymphoma; this class of drug could have application in this
population.
Epigenetic modulators: Bromodomain and Extra-terminal
(BET) inhibitors
BRD4 is a member of the BET family of proteins, which
also includes BRD2, BRD3 and BRDT. These proteins influence
transcription through binding to acetylated histones [99]. BRD4
gained clinical attention after it was described as a partner in the
(15;19)-associated fusion oncogene in an aggressive squamous
carcinoma called NUT midline carcinoma [100]. While screening for
epigenetic regulators in an MLL-induced AML mouse model, Zuber
et al. [101] identified this same protein as being critically required
for disease maintenance. In the same study, suppression of BRD4
by JQ1, the first in class small-molecule BET inhibitor, led to robust
anti-proliferative effects both in vitro and in vivo. JQ1 also led to
down-regulation of MYC transcription and similar activity was seen
in xenograft models of Burkitt lymphoma [102]. In addition BET
inhibition also down-regulated nuclear factor κB (NF-κB) expression
on target genes [103]. Thus, this has generated significant clinical
interest as potential therapeutic strategy for c-MYC and NF-κB driven
hematological malignancies particularly NHLs. The combination of
BET inhibitors and Bruton tyrosine kinase (BTK) inhibitor ibrutinib
decreased the growth of ABC DLBCL tumor (relies heavily on NF-κB
signaling) in mouse models [104].
OTX015 (MK-8628), a new oral BET inhibitor in early clinical
development, showed wide preclinical activity in lymphoma models
and demonstrated synergism with several anticancer agents, especially
with mTOR and BTK inhibitors [105]. A dose escalation open-label
Phase 1 study of this agent which enrolled 45 patients (age range 55-
72 years) with hematological malignancies (mostly lymphomas) was
recently completed. The most common toxic effects reported were
thrombocytopenia (96%) and anemia (91%). With the exception of
thrombocytopenia (56%), grade 3-4 adverse events were infrequent
[106]. A phase 1 study evaluating CPI-0610, an oral BET inhibitor,
in adult patients with progressive lymphoma (NCT01949883) is
currently ongoing and recruiting patients.
Targeting BTK pathway
A subset of B-cell malignancies are dependent on signals from the
B-cell antigen receptor which is mediated through the BTK pathway
[107-109]. Ibrutinib is a small molecule irreversible inhibitor of
BTK. In a phase 1 study, ORR of 60% was observed in a cohort that
included relapsed or refractory NHL and CLL. Although varied,
these responses were observed across the different histology subtypes
including DLBCL [110]. Similar to lenalidomide, ibrutinib appeared
to have more activity in ABC-DLBCL (ORR 40% versus 5% in
the GCB subtype) and thus may have limited utility in AYA NHL
patients [111-114]. A study comparing R-CHOP plus ibrutinib versus
R-CHOP alone in non-GCB DLBCL is ongoing.
Proteasome inhibitors
Proteasome inhibitors (PI) are able to induce apoptosis in
malignant lymphoid cells through inhibition of NF-κB activity
[115]. Several other mechanisms have been elucidated including the
induction of pro-apoptotic Bcl-2 family proteins as well as caspaseindependent
non-apoptotic cell death [116,117]. Bortezomib was
the first PI to be evaluated in clinical trials. Following its success in
multiple myeloma, Fisher et al. [118] showed that this agent had
moderate activity in relapsed/refractory mantle cell lymphoma
(MCL), with both durable responses and tolerable toxicities. Their
observations led to the FDA approval of bortezomib in treatment of
relapsed/refractory MCL.
Bortezomib has also been evaluated in other NHLs. Given that
NF-κB pathway is constitutively activated in ABC DLBCL, it was
rational to suppose bortezomib would provide a clinical benefit in
this disease. Combined with R-CHOP, it improved outcomes in
previously untreated patients with this disease subtype [119], but
only demonstrated minimal clinical activity in heavily pre-treated
DLBCL patients [120,121]. Also, a recently published Phase 2 study
substituting bortezomib (velcade) for vincristine in front-line-
R-CHOP (VR-CAP) showed that VR-CAP did not improve efficacy
vs R-CHOP in non-GCB DLBCL [122]. The Cancer and Leukemia
Group B (CALGB) conducted a multi-institutional Phase 2 trial
of single agent bortezomib in patients with relapsed or refractory
classical HL and did not observe any responses [123].
Carfilzomib is a PI, structurally and mechanically distinct from
bortezomib. A Phase 1/2 study combining this agent with rituximab,
ifosfamide, carboplatin and etoposide (R-ICE) in relapsed/refractory
DLBCL (NCT01959698) is currently enrolling patients. PIs have
been combined with other novel agents in clinical trials. Tan et
al. [109] reported that the combination of bortezomib and the
HDACi panobinostat in relapsed/refractory PTCL was safe and
had encouraging activity (ORR of 43%). Currently, the phase 1 trial
(NCT02142530) is evaluating the combination of carfilzomib and the
HDACi Belinostat in relapsed/refractory NHL.
Apoptotic Pathway: BcL-2 inhibitors
B-cell lymphoma 2 (bcl-2) is a key regulator of apoptosis in
cancer cells and is over expressed in hematologic malignancies,
including NHL [124]. Venetoclax (ABT-199) is a selective, oral Bcl-2
inhibitor whose impressive activity in CLL [125], led to its approval
in CLL patients with 17p deletion. As a single agent, it only showed
modest activity in relapsed/refractory DLBCL (ORR 18%) [112],
although activity was improved in combination with bendamustine
and rituximab (ORR 46%) [126]. Trials evaluating the combination
of venetoclax with other agents; obinutuzumab and polatuzumab
(NCT02611323), and ibrutinib (NCT02419560) are ongoing.
Conclusion
Multiple promising therapies are emerging which may improve outcomes in patients with relapsed/refractory lymphomas. Understanding the biology of lymphomas in AYAs is critical to the effective application of novel treatment strategies. Future trials in relapsed/refractory lymphomas should be driven by disease biology and efforts should continue to be focused on improving the participation of adolescents and young adults in clinical trials.
References
- Lewis DR, Seibel NL, Smith AW, Stedman MR. Adolescent and young adult cancer survival. J Natl Cancer Inst Monogr. 2014; 2014: 228-235.
- Jaglowski SM, Linden E, Termuhlen AM, Flynn JM. Lymphoma in adolescents and young adults. Semin Oncol. 2009; 36: 381-418.
- von Tresckow B, Engert A. Refractory Hodgkin lymphoma. Current opinion in oncology. 2013; 25: 463-469.
- Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol. 2012; 30: 2183-2189.
- Jaffe ES. The 2008 WHO classification of lymphomas: implications for clinical practice and translational research. Hematology Am Soc Hematol Educ Program. 2008; 2009: 523-531.
- Hochberg J, Waxman IM, Kelly KM, Morris E, Cairo MS. Adolescent non-Hodgkin lymphoma and Hodgkin lymphoma: state of the science. Br J Haematol. 2009; 144: 24-40.
- Oschlies I, Klapper W, Zimmermann M, Krams M, Wacker HH, Burkhardt B, et al. Diffuse large B-cell lymphoma in pediatric patients belongs predominantly to the germinal-center type B-cell lymphomas: a clinicopathologic analysis of cases included in the German BFM (Berlin-Frankfurt-Munster) Multicenter Trial Blood. 2006; 107: 4047-4052.
- Gisselbrecht C, Schmitz N, Mounier N, Singh Gill D, Linch DC, Trneny M, et al. Rituximab maintenance therapy after autologous stem-cell transplantation in patients with relapsed CD20(+) diffuse large B-cell lymphoma: final analysis of the collaborative trial in relapsed aggressive lymphoma. J Clin Oncol. 2012; 30: 4462-4469.
- Armand P, Welch S, Kim HT, LaCasce AS, Jacobsen ED, Davids MS, et al. Prognostic factors for patients with diffuse large B cell lymphoma and transformed indolent lymphoma undergoing autologous stem cell transplantation in the positron emission tomography era. Br J Haematol. 2013; 160: 608-617.
- Devillier R, Coso D, Castagna L, Brenot Rossi I, Anastasia A, Chiti A, et al. Positron emission tomography response at the time of autologous stem cell transplantation predicts outcome of patients with relapsed and/or refractory Hodgkin's lymphoma responding to prior salvage therapy. Haematologica. 2012; 97: 1073-1079.
- Jabbour E, Hosing C, Ayers G, Nunez R, Anderlini P, Pro B, et al. Pretransplant positive positron emission tomography/gallium scans predict poor outcome in patients with recurrent/refractory Hodgkin lymphoma. Cancer. 2007; 109: 2481-2489.
- Moskowitz AJ, Yahalom J, Kewalramani T, Maragulia JC, Vanak JM, Zelenetz AD, et al. Pretransplantation functional imaging predicts outcome following autologous stem cell transplantation for relapsed and refractory Hodgkin lymphoma. Blood. 2010; 116: 4934-4937.
- Sehn LH, Fenske TS, Laport GG. Follicular lymphoma: prognostic factors, conventional therapies, and hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012; 18: S82-91.
- Pellegrini C, Dodero A, Chiappella A, Monaco F, Degl'Innocenti D, Salvi F, et al. A phase II study on the role of gemcitabine plus romidepsin (GEMRO regimen) in the treatment of relapsed/refractory peripheral T-cell lymphoma patients. Journal of hematology & oncology. 2016; 9: 38.
- Bleyer WA. Cancer in older adolescents and young adults: epidemiology, diagnosis, treatment, survival, and importance of clinical trials. Med Pediatr Oncol. 2002; 38: 1-10.
- Fernandez CV, Barr RD. Adolescents and young adults with cancer: An orphaned population. Paediatric child health. 2006; 11: 103-106.
- Sender L, Zabokrtsky KB. Adolescent and young adult patients with cancer: a milieu of unique features. Nat Rev Clin Oncol. 2015; 12: 465-480.
- Bleyer A, Budd T, Montello M. Adolescents and young adults with cancer: the scope of the problem and criticality of clinical trials. Cancer. 2006; 107: 1645-1655.
- Liu L, Krailo M, Reaman GH, Bernstein L. Childhood cancer patients' access to cooperative group cancer programs: a population-based study. Cancer. 2003; 97: 1339-1345.
- Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002; 346: 235-242.
- Salles G, Seymour JF, Offner F, Lopez-Guillermo A, Belada D, Xerri L, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet. 2011; 377: 42-51.
- Gabellier L, Cartron G. Obinutuzumab for relapsed or refractory indolent non-Hodgkin's lymphomas. Ther Adv Hematol. 2016; 7: 85-93.
- Morschhauser FA, Cartron G, Thieblemont C, Solal-Celigny P, Haioun C, Bouabdallah R, et al. Obinutuzumab (GA101) monotherapy in relapsed/refractory diffuse large b-cell lymphoma or mantle-cell lymphoma: results from the phase II GAUGUIN study. J Clin Oncol. 2013; 31: 2912-2919.
- Salles GA, Morschhauser F, Solal-Celigny P, Thieblemont C, Lamy T, Tilly H, et al. Obinutuzumab (GA101) in patients with relapsed/refractory indolent non-Hodgkin lymphoma: results from the phase II GAUGUIN study. J Clin Oncol. 2013; 31: 2920-2926.
- Radford J, Davies A, Cartron G, Morschhauser F, Salles G, Marcus R, et al. Obinutuzumab (GA101) plus CHOP or FC in relapsed/refractory follicular lymphoma: results of the GAUDI study (BO21000). Blood. 2013; 122: 1137-1143.
- Teeling JL, French RR, Cragg MS, van den Brakel J, Pluyter M, Huang H, et al. Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood. 2004; 104: 1793-1800.
- Barth MJ, Hernandez-Ilizaliturri FJ, Mavis C, Tsai PC, Gibbs JF, Deeb G, et al. Ofatumumab demonstrates activity against rituximab-sensitive and -resistant cell lines, lymphoma xenografts and primary tumour cells from patients with B-cell lymphoma. Br J Haematol. 2012; 156: 490-498.
- Coiffier B, Lepretre S, Pedersen LM, Gadeberg O, Fredriksen H, van Oers MH, et al. Safety and efficacy of ofatumumab, a fully human monoclonal anti-CD20 antibody, in patients with relapsed or refractory B-cell chronic lymphocytic leukemia: a phase 1-2 study. Blood. 2008; 111: 1094-1100.
- Lemery SJ, Zhang J, Rothmann MD, Yang J, Earp J, Zhao H, et al. U.S. Food and Drug Administration approval: ofatumumab for the treatment of patients with chronic lymphocytic leukemia refractory to fludarabine and alemtuzumab. Clin Cancer Res. 2010; 16: 4331-4338.
- Coiffier B, Radford J, Bosly A, Martinelli G, Verhoef G, Barca G, et al. A multicentre, phase II trial of ofatumumab monotherapy in relapsed/progressive diffuse large B-cell lymphoma. Br J Haematol. 2013; 163: 334-342.
- Witzig TE, Tomblyn MB, Misleh JG, Kio EA, Sharkey RM, Wegener WA, et al. Anti-CD22 90Y-epratuzumab tetraxetan combined with anti-CD20 veltuzumab: a phase I study in patients with relapsed/refractory, aggressive non-Hodgkin lymphoma. Haematologica. 2014; 99: 1738-1745.
- Hamadani M FM, Bello CM, Kipps TJ, Offner F, Verhoef G, Federico M, et al. Safety Profile and Clinical Response To MEDI-551, a Humanized Monoclonal Anti-CD19, In a Phase 1/2 Study In Adults With Relapsed Or Refractory Advanced B-Cell Malignancies. Blood. 2013 122:1810.
- Okeley NM, Miyamoto JB, Zhang X, Sanderson RJ, Benjamin DR, Sievers EL, et al. Intracellular activation of SGN-35, a potent anti-CD30 antibody-drug conjugate. Clin Cancer Res. 2010; 16: 888-897.
- Sasse S, Rothe A, Goergen H, Eichenauer DA, Lohri A, Kreher S, et al. Brentuximab vedotin (SGN-35) in patients with transplant-naive relapsed/refractory Hodgkin lymphoma. Leuk lymphoma. 2013; 54: 2144-2148.
- Garciaz S, Coso D, Peyrade F, Furst S, Duran S, Chetaille B, et al. Brentuximab vedotin followed by allogeneic transplantation as salvage regimen in patients with relapsed and/or refractory Hodgkin's lymphoma. Hematological oncology. 2014; 32: 187-191.
- Salihoglu A, Elverdi T, Karadogan I, Paydas S, Ozdemir E, Erdem G, et al. Brentuximab vedotin for relapsed or refractory Hodgkin lymphoma: experience in Turkey. Ann hematol. 2015; 94: 415-420.
- Pro B, Advani R, Brice P, Bartlett NL, Rosenblatt JD, Illidge T, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol. 2012; 30: 2190-2196.
- Ann S. LaCasce GB, Sawas A, Caimi PF, Agura E, Matous J, Ansell S, et al. Brentuximab Vedotin Plus Bendamustine: A Highly Active Salvage Treatment Regimen for Patients with Relapsed or Refractory Hodgkin Lymphoma. Blood. 2015; 126: 3982.
- Jacobsen ED, Sharman JP, Oki Y, Advani RH, Winter JN, Bello CM, et al. Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory DLBCL with variable CD30 expression. Blood. 2015; 125: 1394-1402.
- Dada R, Zekri J, Al Saadi R. Brentuximab vedotin in pretreated Hodgkin lymphoma patients: a systematic review and meta-analysis. Expert opin biol ther. 2016; 16: 739-745.
- Palanca-Wessels MC, Czuczman M, Salles G, Assouline S, Sehn LH, Flinn I, et al. Safety and activity of the anti-CD79B antibody-drug conjugate polatuzumab vedotin in relapsed or refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukaemia: a phase 1 study. Lancet Oncol. 2015; 16: 704-715.
- Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol. 2015; 33: 1974-1982.
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010; 363: 711-723.
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013; 369: 122-133.
- Armand P. Immune checkpoint blockade in hematologic malignancies. Blood. 2015; 125: 3393-3400.
- Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 2015; 372: 311-319.
- Younes A, Santoro A, Shipp M, Zinzani PL, Timmerman JM, Ansell S, et al. Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016; 17: 1283-1294.
- Armand P, Shipp MA, Ribrag V, Michot JM, Zinzani PL, Kuruvilla J, et al. Programmed Death-1 Blockade With Pembrolizumab in Patients With Classical Hodgkin Lymphoma After Brentuximab Vedotin Failure. J Clin Oncol. 2016.
- Lesokhin AM AS, Armand P, Scott EC, Halwani A, Cohen AD, Lebovic D, et al. Preliminary results of a phase I study of nivolumab (BMS-936558) in patients with relapsed or refractory lymphoid malignancies. Blood. 2014; 124: 291
- Ansell SM, Hurvitz SA, Koenig PA, LaPlant BR, Kabat BF, Fernando D, et al. Phase I study of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2009; 15: 6446-6453.
- Westin JR, Chu F, Zhang M, Fayad LE, Kwak LW, Fowler N, et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol. 2014; 15: 69-77.
- Sadelain M1, Rivière I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003; 3: 35-45.
- Brentjens RJ, Riviere I, Park JH, Davila ML, Wang X, Stefanski J, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011; 118: 4817-4828.
- Davila ML, Sadelain M. Biology and clinical application of CAR T cells for B cell malignancies. Int J Hematol. 2016; 104: 6-17.
- Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012; 119: 2709-2720.
- Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010; 116: 4099-4102.
- Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013; 368: 1509-1518.
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015; 385: 517-528.
- Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015; 33: 540-549.
- Schuster SJ, Nasta SD, Chong EA, Mato AR, Chew A, Shah GD, et al. Sustained Remissions Following Chimeric Antigen Receptor Modified T Cells Directed Against CD19 (CTL019) in Patients with Relapsed or Refractory CD19+ Lymphomas. Blood. 2015; 126: 183.
- Turtle CJ BC, Sommermeyer D, Berger D, Pender B, Melville K, Chaney C, et al. Anti-CD19 Chimeric Antigen Receptor-Modified T Cell Therapy for B Cell Non-Hodgkin Lymphoma and Chronic Lymphocytic Leukemia: Fludarabine and Cyclophosphamide Lymphodepletion Improves In Vivo Expansion and Persistence of CAR-T Cells and Clinical Outcomes. Blood. 2015; 126: 184.
- Ramos CA, Heslop HE, Bernner MK. CAR-T Cell Therapy for Lymphoma. Annu Rev Med. 2016; 67: 165-183.
- Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008; 321: 974-977.
- Topp MS, Gokbuget N, Stein AS, Zugmaier G, O'Brien S, Bargou RC, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015; 16: 57-66.
- Topp MS, Gökbuget N, Zugmaier G, Degenhard E, Goebeler ME, Klinger M, et al. Long-term follow-up of hematologic relapse-free survival in a phase 2 study of blinatumomab in patients with MRD in B-lineage ALL. Blood. 2012; 120: 5185-5187.
- Topp MS, Gokbuget N, Zugmaier G, Klappers P, Stelljes M, Neumann S, et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol. 2014; 32: 4134-4140.
- Topp MS, Kufer P, Gokbuget N, Goebeler M, Klinger M, Neumann S, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011; 29: 2493-2498.
- Goebeler ME, Knop S, Viardot A, Kufer P, Topp MS, Einsele H, et al. Bispecific T-Cell Engager (BiTE) Antibody Construct Blinatumomab for the Treatment of Patients With Relapsed/Refractory Non-Hodgkin Lymphoma: Final Results From a Phase I Study. J Clin Oncol. 2016; 34: 1104-1111.
- Kronke J, Udeshi ND, Narla A, Grauman P, Hurst SN, McConkey M, et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science. 2014; 343: 301-305.
- Witzig TE, Vose JM, Zinzani PL, Reeder CB, Buckstein R, Polikoff JA, et al. An international phase II trial of single-agent lenalidomide for relapsed or refractory aggressive B-cell non-Hodgkin's lymphoma. Ann Oncol. 2011; 22: 1622-1627.
- Hernandez-Ilizaliturri FJ, Deeb G, Zinzani PL, Pileri SA, Malik F, Macon WR, et al. Higher response to lenalidomide in relapsed/refractory diffuse large B-cell lymphoma in nongerminal center B-cell-like than in germinal center B-cell-like phenotype. Cancer. 2011; 117: 5058-5066.
- Zelenetz AD. Emerging treatment options for B-cell lymphomas. J Natl Compr Canc Netw. 2015; 13: 666-669.
- Fehniger TA, Larson S, Trinkaus K, Siegel MJ, Cashen AF, Blum KA, et al. A phase 2 multicenter study of lenalidomide in relapsed or refractory classical Hodgkin lymphoma. Blood. 2011; 118: 5119-5125.
- Panwalkar A, Verstovsek S, Giles FJ. Mammalian target of rapamycin inhibition as therapy for hematologic malignancies. Cancer. 2004; 100: 657-666.
- Jiang BH, Liu LZ. Role of mTOR in anticancer drug resistance: perspectives for improved drug treatment. Drug Resist Updat. 2008; 11: 63-76.
- Witzig TE, Reeder CB, LaPlant BR, Gupta M, Johnston PB, Micallef IN, et al. A phase II trial of the oral mTOR inhibitor everolimus in relapsed aggressive lymphoma. Leukemia. 2011; 25: 341-347.
- Barnes JA, Jacobsen E, Feng Y, Freedman A, Hochberg EP, LaCasce AS, et al. Everolimus in combination with rituximab induces complete responses in heavily pretreated diffuse large B-cell lymphoma. Haematologica. 2013; 98: 615-619.
- Johnston PB, LaPlant B, McPhail E, Habermann TM, Inwards DJ, Micallef IN, et al. Everolimus combined with R-CHOP-21 for new, untreated, diffuse large B-cell lymphoma (NCCTG 1085 [Alliance]): safety and efficacy results of a phase 1 and feasibility trial. Lancet Haematol. 2016; 3: e309-316.
- Johnston PB, Inwards DJ, Colgan JP, Laplant BR, Kabat BF, Habermann TM, et al. A Phase II trial of the oral mTOR inhibitor everolimus in relapsed Hodgkin lymphoma. Am J Hematol. 2010; 85: 320-324.
- Lemoine M, Derenzini E, Buglio D, Medeiros LJ, Davis RE, Zhang J, et al. The pan-deacetylase inhibitor panobinostat induces cell death and synergizes with everolimus in Hodgkin lymphoma cell lines. Blood. 2012; 119: 4017-4025.
- Oki Y, Buglio D, Fanale M, Fayad L, Copeland A, Romaguera J, et al. Phase I study of panobinostat plus everolimus in patients with relapsed or refractory lymphoma. Clin Cancer Res. 2013; 19: 6882-6890.
- Apuri S, Sokol L. An overview of investigational Histone deacetylase inhibitors (HDACis) for the treatment of non-Hodgkin's lymphoma. Expert Opin Investig Drugs. 2016; 25: 687-696.
- Younes A, Sureda A, Ben-Yehuda D, Zinzani PL, Ong TC, Prince HM, et al. Panobinostat in patients with relapsed/refractory Hodgkin's lymphoma after autologous stem-cell transplantation: results of a phase II study. J Clin Oncol. 2012; 30: 2197-2203.
- Harrison SJ, Hsu AK, Neeson P, Younes A, Sureda A, Engert A, et al. Early thymus and activation-regulated chemokine (TARC) reduction and response following panobinostat treatment in patients with relapsed/refractory Hodgkin lymphoma following autologous stem cell transplant. Leuk lymphoma. 2014; 55: 1053-1060.
- Kirschbaum M, Frankel P, Popplewell L, Zain J, Delioukina M, Pullarkat V, et al. Phase II study of vorinostat for treatment of relapsed or refractory indolent non-Hodgkin's lymphoma and mantle cell lymphoma. J Clin Oncol. 2011; 29: 1198-1203.
- Younes A, Oki Y, Bociek RG, Kuruvilla J, Fanale M, Neelapu S, et al. Mocetinostat for relapsed classical Hodgkin's lymphoma: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2011; 12: 1222-1228.
- Coiffier B, Pro B, Prince HM, Foss F, Sokol L, Greenwood M, et al. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol. 2012; 30: 631-636.
- O'Connor OA, Horwitz S, Masszi T, Van Hoof A, Brown P, Doorduijn J, et al. Belinostat in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma: Results of the Pivotal Phase II BELIEF (CLN-19) Study. J Clin Oncol. 2015; 33: 2492-2499.
- Crump M, Coiffier B, Jacobsen ED, Sun L, Ricker JL, Xie H, et al. Phase II trial of oral vorinostat (suberoylanilide hydroxamic acid) in relapsed diffuse large-B-cell lymphoma. Ann Oncol. 2008; 19: 964-969.
- Bodo J, Zhao X, Sharma A, Hill BT, Portell CA, Lannutti BJ, et al. The phosphatidylinositol 3-kinases (PI3K) inhibitor GS-1101 synergistically potentiates histone deacetylase inhibitor-induced proliferation inhibition and apoptosis through the inactivation of PI3K and extracellular signal-regulated kinase pathways. BJ Haematol. 2013; 163: 72-80.
- Dasmahapatra G, Lembersky D, Kramer L, Fisher RI, Friedberg J, Dent P, et al. The pan-HDAC inhibitor vorinostat potentiates the activity of the proteasome inhibitor carfilzomib in human DLBCL cells in vitro and in vivo. Blood. 2010; 115: 4478-4487.
- Montanari F, Diefenbach C. Relapsed Hodgkin lymphoma: management strategies. Curr Hematol Malig Rep. 2014; 9: 284-293.
- Younes A, Berdeja JG, Patel MR, Flinn I, Gerecitano JF, Neelapu SS, et al. Safety, tolerability, and preliminary activity of CUDC-907, a first-in-class, oral, dual inhibitor of HDAC and PI3K, in patients with relapsed or refractory lymphoma or multiple myeloma: an open-label, dose-escalation, phase 1 trial. Lancet Oncol. 2016.
- Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002; 298: 1039-1043.
- Berg T, Thoene S, Yap D, Wee T, Schoeler N, Rosten P, et al. A transgenic mouse model demonstrating the oncogenic role of mutations in the polycomb-group gene EZH2 in lymphomagenesis. Blood. 2014; 123: 3914-3924.
- Bödör C, Grossmann V, Popov N, Okosun J, O'Riain C, Tan K, et al. EZH2 mutations are frequent and represent an early event in follicular lymphoma. Blood. 2013; 122: 3165-3168.
- Morin RD, Johnson NA, Severson TM, Mungall AJ, An J, Goya R, et al. Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat genet. 2010; 42: 181-185.
- Knutson SK, Kawano S, Minoshima Y, Warholic NM, Huang KC, Xiao Y, et al. Selective inhibition of EZH2 by EPZ-6438 leads to potent antitumor activity in EZH2-mutant non-Hodgkin lymphoma. Mol cancer ther. 2014; 13: 842-854.
- Wu SY, Chiang CM. The double bromodomain-containing chromatin adaptor Brd4 and transcriptional regulation. J Biol Chem. 2007; 282: 13141-13145.
- French CA, Miyoshi I, Aster JC, Kubonishi I, Kroll TG, Dal Cin P, et al. BRD4 bromodomain gene rearrangement in aggressive carcinoma with translocation t(15;19). Am j pathol. 2001; 159: 1987-1992.
- Zuber J, Shi J, Wang E, Rappaport AR, Herrmann H, Sison EA, et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011; 478: 524-528.
- Mertz JA, Conery AR, Bryant BM, Sandy P, Balasubramanian S, Mele DA, et al. Targeting MYC dependence in cancer by inhibiting BET bromodomains. Proc Natl Acad Sci U S A. 2011; 108: 16669-16674.
- Nicodeme E, Jeffrey KL, Schaefer U, Beinke S, Dewell S, Chung CW, et al. Suppression of inflammation by a synthetic histone mimic. Nature. 2010; 468: 1119-1123.
- Ceribelli M, Kelly PN, Shaffer AL, Wright GW, Xiao W, Yang Y, et al. Blockade of oncogenic IkappaB kinase activity in diffuse large B-cell lymphoma by bromodomain and extraterminal domain protein inhibitors. Proc Natl Acad Sci U S A. 2014; 111: 11365-11370.
- Boi M, Gaudio E, Bonetti P, Kwee I, Bernasconi E, Tarantelli C, et al. The BET Bromodomain Inhibitor OTX015 Affects Pathogenetic Pathways in Preclinical B-cell Tumor Models and Synergizes with Targeted Drugs. Clin Cancer Res. 2015; 21: 1628-1638.
- Amorim S, Stathis A, Gleeson M, Iyengar S, Magarotto V, Leleu X, et al. Bromodomain inhibitor OTX015 in patients with lymphoma or multiple myeloma: a dose-escalation, open-label, pharmacokinetic, phase 1 study. Lancet Haematol. 2016; 3: e196-204.
- Ogura M, Ando K, Suzuki T, Ishizawa K, Oh SY, Itoh K, et al. A multicentre phase II study of vorinostat in patients with relapsed or refractory indolent B-cell non-Hodgkin lymphoma and mantle cell lymphoma. Br J haematol. 2014; 165: 768-776.
- Ohmachi K, Niitsu N, Uchida T, Kim SJ, Ando K, Takahashi N, et al. Multicenter phase II study of bendamustine plus rituximab in patients with relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2013; 31: 2103-2109.
- Tan D, Phipps C, Hwang WY, Tan SY, Yeap CH, Chan YH, et al. Panobinostat in combination with bortezomib in patients with relapsed or refractory peripheral T-cell lymphoma: an open-label, multicentre phase 2 trial. Lancet Haematol. 2015; 2: e326-333.
- Advani RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. 2013; 31: 88-94.
- Wilson WH, Young RM, Schmitz R, Yang Y, Pittaluga S, Wright G, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med. 2015; 21: 922-926.
- Gerecitano JF RA, Seymour JF, Roberts AW, Kahl BS, Pagel JM, Puvvada S, et al. A phase 1 study of venetoclax (ABT-199/GDC-0199) monotherapy in patients with relapsed/refractory non-Hodgkin lymphoma. Blood. 2015; 126: 254.
- Gururajan M, Jennings CD, Bondada S. Cutting edge: constitutive B cell receptor signaling is critical for basal growth of B lymphoma. J Immunol. 2006; 176: 5715-5719.
- Küppers R. Mechanisms of B-cell lymphoma pathogenesis. Nat Rev Cancer. 2005; 5: 251-262.
- O'Connor OA, Czuczman MS. Novel approaches for the treatment of NHL: Proteasome inhibition and immune modulation. Leuk Lymphoma. 2008; 49: 59-66.
- Olejniczak SH, Blickwedehl J, Belicha-Villanueva A, Bangia N, Riaz W, Mavis C, et al. Distinct molecular mechanisms responsible for bortezomib-induced death of therapy-resistant versus -sensitive B-NHL cells. Blood. 2010; 116: 5605-5614.
- Perez-Galan P, Roue G, Villamor N, Montserrat E, Campo E, Colomer D. The proteasome inhibitor bortezomib induces apoptosis in mantle-cell lymphoma through generation of ROS and Noxa activation independent of p53 status. Blood. 2006; 107: 257-264.
- Fisher RI, Bernstein SH, Kahl BS, Djulbegovic B, Robertson MJ, de Vos S, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006; 24: 4867-4874.
- Dunleavy K, Pittaluga S, Czuczman MS, Dave SS, Wright G, Grant N, et al. Differential efficacy of bortezomib plus chemotherapy within molecular subtypes of diffuse large B-cell lymphoma. Blood. 2009; 113: 6069-6076.
- Elstrom RL, Andemariam B, Martin P, Ruan J, Shore TB, Coleman M, et al. Bortezomib in combination with rituximab, dexamethasone, ifosfamide, cisplatin and etoposide chemoimmunotherapy in patients with relapsed and primary refractory diffuse large B-cell lymphoma. Leuk Lymphoma. 2012; 53: 1469-1473.
- Evens AM, Rosen ST, Helenowski I, Kline J, Larsen A, Colvin J, et al. A phase I/II trial of bortezomib combined concurrently with gemcitabine for relapsed or refractory DLBCL and peripheral T-cell lymphomas. Br J Haematol. 2013; 163: 55-61.
- Offner F, Samoilova O, Osmanov E, Eom HS, Topp MS, Raposo J, et al. Frontline rituximab, cyclophosphamide, doxorubicin, and prednisone with bortezomib (VR-CAP) or vincristine (R-CHOP) for non-GCB DLBCL. Blood. 2015; 126: 1893-1901.
- Blum KA, Johnson JL, Niedzwiecki D, Canellos GP, Cheson BD, Bartlett NL. Single agent bortezomib in the treatment of relapsed and refractory Hodgkin lymphoma: cancer and leukemia Group B protocol 50206. Leuk Lymphoma. 2007; 48: 1313-1319.
- Tsujimoto Y, Shimizu S. Bcl-2 family: life-or-death switch. FEBS Lett. 2000; 466: 6-10.
- Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, et al. Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med. 2016; 374: 311-322.
- de Vos S, Swinnen L, Kozloff M, Wang D, Reid E, Zhu M, et al. A Dose-escalation study of venetoclax (ABT-199/GDC-0199) in combination with bendamustine and rituximab in patients with relapsed or refractory non-Hodgkin’s lymphoma. Blood. 2015; 126: 255.