Case Report
Targeted Therapy’s Skin Toxicities after Immunotherapy: Skin Toxicity of Tyrosine Kinase Inhibitor Therapy Following Nilovumab Therapy for Metastatic Renal Cell Carcinoma
Benjamin Verret1, Cécile Badoual2,3, Pierre Combe1,2, Agnes Carlotti4 and Stephane Oudard1,3*
1Hôpital Européen Georges Pompidou, Oncology Department, 20 rue Leblanc 75015 Paris
2Hôpital Européen Georges Pompidou, Anatomopathology Department, 20 rue Leblanc, 75015, Paris
3Université Paris Descartes, Paris
4Hôpital Cochin, Anatomopathology Depatment, 27-30 Faubourg Saint Jacques, 75014 Paris, France
*Corresponding author: Stéphane Oudard, Service d’oncologie médicale, Hopital Européen Georges Pompidou, 20 rue Leblanc, 75015 Paris, France
Published: 27 Oct, 2016
Cite this article as: Verret B, Badoual C, Combe P, Carlotti
A, Oudard S. Targeted Therapy’s Skin
Toxicities after Immunotherapy: Skin
Toxicity of Tyrosine Kinase Inhibitor
Therapy Following Nilovumab Therapy
for Metastatic Renal Cell Carcinoma.
Clin Oncol. 2016; 1: 1133.
Abstract
Anti-programmed cell death antibodies (anti-PD-1) are currently under development for the
treatment of Metastatic Renal Cell Carcinoma (mRCC) and safety of subsequent treatment by
VEGFR Tyrosine Kinase Inhibitors (TKI) is not known. We report two cases of unexpectedly severe
skin rashes occurring on initiation of treatment by the tyrosine kinase inhibitor sorafenib after prior
treatment with the anti-PD-1 nivolumab. Attention is drawn to the potential skin toxicity of TKI
treatment after anti-PD-1 administration.
Keywords: Immunotherapy; Anti-pd1; Nivolumab; Sorafenib; Renal cell carcinoma; Skin
rashes; Drugs sequence
Abbreviations
Anti-PD-1: Anti-Programmed Cell Death Antibodies; mRCC: Metastatic Renal Cell Carcinoma; TKI: Tyrosine Kinase Inhibitor; VEGFR: Vascular Endothelial Growth Factor Receptor; PDGFR: Platelet-Derived Growth Factor Receptor; PFS: Progression-Free Survival; OS: Overall Survival; mTOR: Mammalian Target of Rapamycin
Introduction
The customary first-line treatment of mRCC in 70% of cases is the TKI sunitinib, an inhibitor of Vascular Endothelial Growth Factor Receptor (VEGFR) and Platelet-Derived Growth Factor Receptor (PDGFR), which has been shown to significantly improve Progression-Free Survival (PFS) and Overall Survival (OS) [1,2]. RCC generally are associated with diminished probability and duration of response in second-line therapies. For this reason, there are substantial clinical research efforts to find more effective therapies including immunotherapies, which can be associated with longer duration of responses compared to targeted therapies. The drugs, in most common secondline use, are the TKI sorafenib, an orally active multikinase inhibitor, which has shown improved PFS and OS after cytokine-based treatment [3], the TKI axitinib and cabozantinib [4], and inhibitors of the mammalian target of rapamycin (mTOR) such as everolimus [5]. In addition, anti-programmed cell death antibodies (anti-PD1, such as Nivolumab) have shown to be active in second-line post- TKI and prolong overall survival with a good toxicity profile and quality of life. We report two cases of unexpectedly severe skin rashes occurring on initiation of treatment by the tyrosine kinase inhibitor (TKI) sorafenib after prior treatment with the anti-PD-1 nivolumab.
Case Presentation
Case 1
A 63-year old man who presented with back pain and hematuria was diagnosed with Fuhrman
Grade III clear-cell RCC and lung metastases. He received the TKI sunitinib (50 mg/day for 4
weeks/6) until radiographic progression at 1 year followed by the anti-PD1 drug nivolumab (3
mg/kg solution intravenously every 2 weeks) (BMS CA209-025 trial) [6] until documented disease
progression at 5 months. The third-line drug was the TKI sorafenib. After just one week of sorafenib
therapy, however, the patient was admitted to intensive care for a
grade 4 skin rash (Figure 1a and b). Cyclosporine was initiated for
suspected toxic epidermal necrolysis. Polymerase chain reaction tests
for cytomegalovirus (CMV), Epstein-Barr virus (EBV), Herpes virus
(HHV6) and parvovirus B19, as well as serology tests for human
immunodeficiency virus (HIV) and syphilis, were negative. A skin
biopsy confirmed epidermal necrolysis and lymphocytic infiltration
of the skin with rare FOXP3 positive cells under the malpighian
epithelium (Figure 1b). Severe toxidermitis was diagnosed. The
patient was discharged from hospital after 7 days. Two months later,
treatment by everolimus was initiated without inducing any severe
side-effects.
Case 2
A 60-year old woman with clear-cell RCC and pancreatic
metastases received first-line TKI therapy (sunitinib, 50 mg/day 4
weeks/6) for 30 months until radiographic progression, followed by
second-line nivolumab (8 infusions of 3 mg/kg every 2 weeks) until
progression at 3 months (BMS CA209-025 trial), followed by thirdline
tasquinimod which had, however, to be discontinued after 5
months because of lung toxicity (dyspnea). Sorafenib treatment was
initiated but, after just 2 weeks, the patient experienced a grade 4 skin
rash and grade 3 hand-foot syndrome (Figure 2). After a 6-month
respite period, treatment was switched to cabozantinib with no severe
adverse effects (METEOR XL184-308 trial) [7].
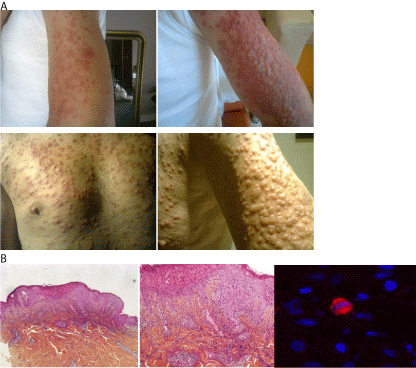
Figure 1
Figure 1
Case 1: Skin toxicity on sorafenib initiation after nivolumab
treatment.
(a) Grade 4 erythematous skin rash at Day 7 on arms and chest.
(b) Skin biopsy showing epidermal necrolysis and lymphocytic infiltration of
the skin. Presence of rare regulating FOXP3 positive cells situated under the
malpighian epithelium.
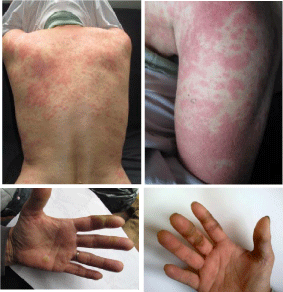
Figure 2
Figure 2
Case 2: Skin toxicity on sorafenib intiation after nivolumab
treatment with skin rash and “hand and foot syndrome”.
Discussion
The recently developed anti-PD-1 nivolumab (BMS-936558)
increases survival and helps to reverse the exhausted phenotype
of T cells expressing PD-1 and has shown promise as second- or
higher-line therapy in patients with advanced non-small-cell lung
cancer, melanoma and RCC [8]. PD-1 is a key immune checkpoint
receptor that is expressed by activated T-cells and mediates
immunosuppression. It functions mainly in peripheral tissues when
T-cells encounter immunosuppressive PD-1 ligands (PD-L1 (B7-
H1) and PD-L2 (B7-DC) expressed by tumor and/or stromal cells. In
experimental studies, inhibition of the interaction between PD-1 and
PD-L1 enhanced T-cell responses and mediated antitumor activity
[9]. The hand-foot skin reaction associated with the TKI sorafenib
is well known. The reported occurrence of skin rash of any grade is
about 40%; with very few cases of grade 3-4 rashes [3]. Grade 4 rashes
resulting in toxic epidermal necrolysis and admission to intensive
care are extremely rare with sorafenib. According to a phase 1 trial of
296 patients, nivolumab has acceptable short-term safety. The most
common adverse events were fatigue, decreased appetite, diarrhea,
nausea, cough, dyspnea, constipation, vomiting, rash, pyrexia, and
headache, with 3 drug-related deaths from grade 5 pneumonitis [8].
Data on its long-term use in the wake of a TKI are, however, scarce.
In a multicenter retrospective study of VEGFR-TKI administration
after PD1/PD-L1 inhibition in 43 patients with mRCC, the median
time to treatment failure was 6.9 months, with no notable toxicity
[10]. However, this study concerned a small size of patient and some
rare adverse events might not be detected.
Neither of our patients had a history of autoimmune or of skin
disease that might have been omened skin toxicity, suggesting that
nivolumab may have contributed toward the onset of the skin toxicity
observed on initiation of sorafenib therapy. As the interval between
nivolumab and sorafenib therapy was 3 weeks or longer, it would seem
that the skin toxicity was due to exacerbation by immune sensitization
rather than cumulative toxicity (Table 1). The rapid onset of the skin
toxicity also suggested that the cause was immunological. We found
rare FOXP3 positive cells under the malpighian epithelium, FOXP3
is a key regulatory gene for the action of T cells [11] and it is one
more argument for an immune mechanism. The exact pathogenesis
remains unknown, but we can hypothesize that nivolumab enhance
T-cell responses against tumor cell but also some unspecific T-cell
responses that can improved immune adverse event and so skin
toxicity of sorafenib. This hypothesis means that this rash was not
caused by a specific T-cell reaction against sorafenib but by an
unspecific immune response triggered by TKI due to prior use of
anti-PD-1.
The rationale of anti-PD-1 therapy is to enhance T-cell responses
to tumor cells but we hypothesize that such therapy might also
trigger unwanted immune responses such as skin toxicity worsening.
Our case reports pinpoint the need to further examine which drug
sequences are most appropriate in patients with mRCC.
Table 1
Table 1
Cases of severe skin toxicity on initiation of TKI treatment after progression on anti-PD-1 treatment.
Acknowledgement
We acknowledge ARTIC (Association pour la recherche de thérapeutiques innovantes en cancérologie) for supporting this work.
References
- Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007; 356: 115–124.
- Heng DYC, Wells JC, Rini BI, Beuselinck B, Lee J-L, Knox JJ, et al. Cytoreductive nephrectomy in patients with synchronous metastases from renal cell carcinoma: results from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur Urol. 2014; 66: 704-710.
- Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007; 356: 125–134.
- Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011; 378: 1931-1939.
- Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma : final results and analysis of prognostic factors. Cancer. 2010; 116 : 4256-4265.
- http://clinicaltrials.gov/show/NCT01668784 (In Press).
- http://clinicaltrials.gov/show/NCT01865747 (In Press).
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med. 2012; 366: 2443–2454.
- Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012; 24: 207‑212.
- Albiges L, Fay AP, Xie W, Krajewski K, McDermott DF, Heng DYC, et al. Efficacy of targeted therapies after PD-1/PD-L1 blockade in metastatic renal cell carcinoma. Eur J Cancer. 2015; 51: 2580-2586.
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003; 299: 1057-1061.