Research Article
Non-Metastatic Small Cell Carcinoma of the Urinary Bladder – Clinical Outcomes from a Single Institution
Jonathan Teo Shunming1, Sim Hong Gee2, Ng Lay Guat1, Toh Chee Keong3, Jonathan Teh Yi Hui4, Khor Li Yan5 and Lee Lui Shiong1*
1Department of Urology, Singapore General Hospital, Singapore
2Gleneagles Medical Centre, Singapore
3Department of Medical Oncology, National Cancer Centre, Singapore
4Department of Radiation Oncology, National Cancer Centre, Singapore
5Department of Pathology, Singapore General Hospital, Singapore
*Corresponding author: Lee Lui Shiong, Department of Urology, Singapore General Hospital, Singapore
Published: 24 Oct, 2016
Cite this article as: Shunming JT, Gee SH, Guat NL,
Keong TC, Hui JTY, Yan KL, et al. Non-
Metastatic Small Cell Carcinoma of the
Urinary Bladder – Clinical Outcomes
from a Single Institution. Clin Oncol.
2016; 1: 1130.
Abstract
Objective: This study aims to ascertain the oncological outcomes of histologically proven nonmetastatic
primary small cell carcinoma of the urinary bladder in a single institution.
Materials and Methods: All suitable patients were identified from a prospectively maintained
cancer registry. The outcomes analysed included demographics, treatment received and survival
outcomes of Overall Survival (OS) and Disease Specific Survival (DSS).The study cohort was also
dichotomised to pure small cell carcinoma and mixed small cell carcinoma for an exploratory
analysis to evaluate the influence of pathological subtypes on DSS and OS.
Results: Thirteen patients were identified to have organ-confined small cell carcinoma of the urinary
bladder. The mean age of these patients was 60 years old at diagnosis. Treatment modalities included
radical cystectomy (n=3), partial cystectomy (n=2), combined chemotherapy and radiotherapy
(n=4), radiotherapy alone (n=2), and no immediate treatment (n=2). At diagnosis, clinical staging
consisted of organ-confined disease cT2 or better (n=11) and cT3/4 disease (n=2). The Overall
Survival (OS) and Disease Specific Survival (DSS) rate of the entire cohort were 13 months and
69.2% at 1 year, 69.2% at 2 years and 61.5% at 5 years respectively.
Out of the 13 patients, 5 had pure small cell carcinoma of the bladder, and 8 had mixed small cell
carcinoma of the bladder. The OS was 10 months for the pure small cell carcinoma group and 97
months for the mixed small cell carcinoma group. The DSS rate was 40% at 1 year and 0% at 2 years
for the pure SCCB group. The DSS rate was 75% at 1 year, 62.5% at 2 years and 37.5% at 5 years for
the mixed SCCB group.
Conclusion: Small cell carcinoma of the urinary bladder has a poor prognosis with aggressive
progression. The presence of conventional urothelial carcinoma in SCCB appears to confer a
better prognosis. This needs validation in prospective studies, and the exact mechanism requires
elucidation.
Keywords: Carcinoma; Small cell; Urinary bladder; Chemotherapy; Surgery; Radiation
Introduction
Extrapulmonary small cell carcinoma is an uncommon neoplasm of the genitourinary tract, of
which the urinary bladder is the most common site [1-4].
Small cell carcinoma of the urinary bladder (SCCB) has many features similar to small cell
carcinoma of the lung, including an aggressive biological behaviour associated with early metastasis,
and variable response to systemic chemotherapy [5]. It is also associated with an advanced stage at
clinical presentation [6] and dismal 5 year survival rates of 8.1% to 16% [7,8].
SCCB accounts for less than 1% of all primary bladder malignancies [9-13], and this condition
has no established best treatment strategy [14].
There have been less than 15 case series, to date, in the English literature, with case numbers
in these reports ranging between 18 to 64 patients per series, with the largest multi-centre series
consisting of 625 patients [15]. These case series comprise a
heterogeneous mix of organ confined disease, locally advanced
disease and metastatic disease, and do not always discriminate
outcomes between the various stages of disease.
This study aims to establish the oncological outcomes following
treatment of histologically proven non-metastatic primary SCCB in a
single institution.
Table 1
Table 2
Materials and Methods
With approval from the institutional ethics review board, all
patients with histologically diagnosed non-metastatic primary
small cell carcinoma of the urinary bladder were identified. Clinical
data was extracted from the Department of Urology, Singapore
General Hospital Urological Cancer Registry, Business Intelligence
Enterprise Edition (Oracle Business Intelligence Enterprise Edition),
and comprised of data captured between 1st January 1990 and 31st
December 2014. Those with existing small cell carcinoma of the lung
or synchronous upper urinary tract tumours were excluded.
All cases were restaged, at data analysis, using the 7th edition
American Joint Committee on Cancer (AJCC) 2010 TNM
classification system for diagnostic uniformity in this study.
The outcomes analysed included baseline characteristics and
demographics, presenting symptoms, clinical stage of disease at
diagnosis, treatment modality received and outcomes of Disease
Specific Survival (DSS) and Overall Survival (OS).
As an exploratory analysis to evaluate the effect of histological
subtypes, we segregated the cohort into 2 sub-groups – those with
pure SCCB and those with mixed SCCB. The cohort of pure SCCB
included patients with only small cell carcinoma on histopathology,
and the cohort with mixed SCCB were defined as those with
conventional urothelial carcinoma admixed with small cell carcinoma
on histopathology.
The statistical analysis comprised the Mann Whitney U test
for continuous variables, and Kaplan Meier analysis for survival
outcomes determination. Statistical significance was defined at p
<0.05 in this study.
Results
There were 14 patients identified with organ confined small cell
bladder carcinoma from the database, and after excluding one patient
with a synchronous upper tract tumour, the number of suitable
patients available for analysis was 13.
The mean follow-up period was 45 months (median 13, range
3–211). There were a total of 10 male and 3 female patients, with a
mean age of 60 years (median 66 years, range 50-85 years. All patients
had macroscopic haematuria as their initial presenting symptom.
There were 9 patients with a history of chronic tobacco usage.
The clinical stage at diagnosis comprised of T2 stage in n=11
and T3/4 disease in n=2. The treatment received consisted of radical
cystectomy (n=3), partial cystectomy (n=2), chemoradiotherapy
(n=4) and radiotherapy (as a single treatment modality) (n=2). There
were two patients that declined immediate therapy (Table 2).
Of those who underwent radical cystectomy and pelvic lymph
node dissection, the pathological stage consisted of pT2N0 (n=2)
and pT4N0 (n=1). These patients declined neoadjuvant therapy
and adjuvant treatment. For patients who underwent combined
chemotherapy and radiotherapy, the chemotherapeutic agents
comprised a combination of etoposide and cisplatin or etoposide and
carboplatin (in those with existing renal impairment). The dosage of
radical radiation administered to the bladder were between 55-66 Gy
in divided fractions.
Of the 2 patients who did not receive immediate treatment, one
patient agreed to receive palliative treatment when symptomatic
from rapid disease progression over 3 months. The other underwent
repeated transurethral resection for bladder outlet obstruction and
bleeding. Both patients had refused initial treatment offered by their
managing physicians.
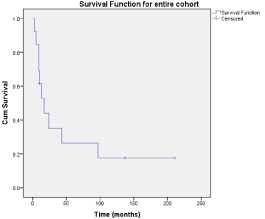
The OS and DSS rate for this patient cohort was 13 months and
69.2% at 1 year, 69.2% at 2 years and 61.5% at 5 years respectively
(Figure 1).
As an exploratory analysis to determine the effect of
histopathological subtype on survival outcomes, we dichotomised
the population into two sub-groups comprising those with mixed
small cell carcinoma histology (n=8) and those with pure small cell
carcinoma histology (n=5).
The mixed SCCB sub-group were younger (median age 60 years,
mean 64, range 50-85) compared to the pure SCCB sub-group (median
age 71 years, mean 69, range 50-83) (Table 1). The mixed sub-group
SCCB comprised 20 percent (2 out of 8) with locally advanced disease
(T3-4), while the pure SCCB sub-group all comprised of organ
confined cancer. There were no major differences in the modality of
treatment received across these 2 sub-groups (Table 2). Despite the
disparity of clinical staging, the overall survival of the mixed SCCB
group was higher than that of the pure SCCB group (Table 1). The OS
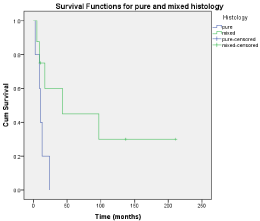
for the pure and mixed SCCB group was 10 months and 97 months
respectively. The DSS rate was 75% at 1 year, 62.5% at 2 years and
37.5% at 5 years for the mixed SCCB group, while corresponding
figures for the pure SCCB group were 40%, 0% and 0% respectively.
The corresponding Kaplan Meier survival curves are shown in Figure
2. The median follow-up period was 97 months (range 5-211 months)
for the mixed SCCB group, while that of the pure SCCB group was
10 months (range 3-24 months) (Figure 3). The difference in followup
duration was largely influenced by early occurrence of mortality
events in the pure SCCB group.
The recurrence rate and median time to recurrence are
summarised in Table 3. All the recurrence were distant metastasis
to brain and bone. Only 1 recurrence from the radiotherapy group
had a local recurrence in the bladder detected together with distant
metastasis.
Figure 1
Figure 2
Figure 3
Figure 3
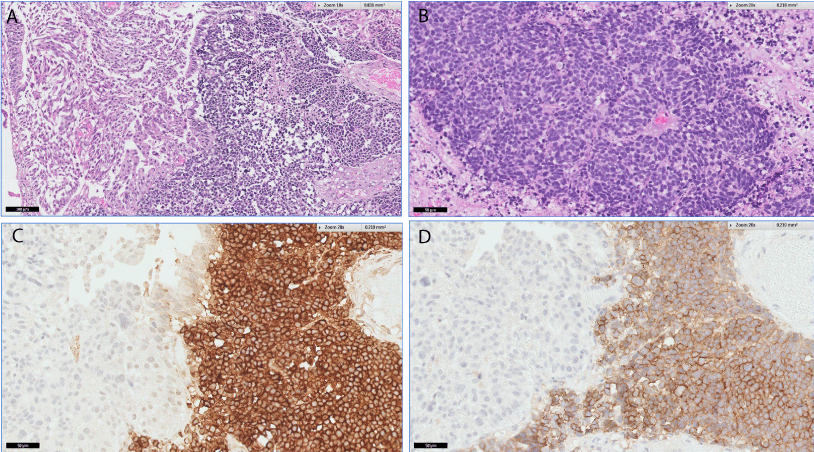
A: H&E staining for mixed SCCB. Urothelial carcinoma admixed
with small cell carcinoma. B: H&E staining for pure SCCB. Solid sheets of
cells, some in rosette formation. C: Synaptophysin staining for mixed SCCB.
Only small cell carcinoma stained with synaptophysin. Urothelial carcinoma
is not stained. D: CD 56 staining for mixed small cell carcinoma. Only small
cell carcinoma staining positive for CD 56.
Discussion
The optimal treatment strategy for this aggressive malignancy
is not clear, although evidence from retrospective data suggests
that neoadjuvant chemotherapy combined with radical surgery is
associated with the most favourable OS [15]. The difference in 3-year
OS is reported to range between 53% in the combination treatment
arm, to 39% for those treated by radical cystectomy alone, and 14%
for those who underwent radical cystectomy followed by adjuvant
chemotherapy. Reported a retrospective series of patients with small
cell carcinoma of the bladder who underwent radical cystectomy
and found that those who underwent adjuvant chemotherapy had
improved 5-year survival compared with those who did not (43%
versus 20%, p=0.03). The patients who had adjuvant chemotherapy
had a higher rate of nodal metastasis than those who did not (61.1%
versus 27.7%, p=0.01). The need for multimodality therapy in SCCB
comprising local and systemic therapy is also emphasized by other
authors [7,9,10,13]. This principle of therapy is also mirrored in the
management of small cell carcinoma in other extra-pulmonary sites,
where the need for combined local and systemic therapy confers
superior survival outcomes [16].
The optimal regime for chemotherapy is not clear for SCCB.
Mukesh et al. [4] reported their series of patients who underwent
chemotherapy either using cyclophosphamide, doxorubicin and
vincristine, or carboplatin with etoposide, or alternative platinumbased
regimes. The chemotherapy regime used in our patients were
etoposide and a platinum based agent, combined with radiotherapy.
Although there are larger reported case series in literature,
these include patients with metastatic disease at presentation. To
our knowledge, the largest reported series [15] was a retrospective
analysis using the National Cancer Database comprising 625 patients.
However, as the data was derived from an administrative dataset,
detailed information about treatment rendered was not available
for analysis. The patients from this existing series included only
individuals with organ-confined disease, and largely comprised those
with clinical stage T1 or T2 at presentation (85% of cohort). The overall
survival was 13 months for the entire study group, however, suggests
that SCCB is a highly aggressive disease, with a short time from
diagnosis to metastasis or death. The treatment received by patients in
this study cohort were varied, and largely reflected the retrospective
nature of the study where patient selection predominated. In the
patients without disease related mortality, the treatment received
included radical cystectomy (n=1), chemoradiotherapy (n=1), and
radical radiotherapy (n=1). The follow-up for the patient who had
radical cystectomy was 211 months, radiotherapy alone 137 months,
and chemoradiotherapy 10 months. There is a suggestion that
neoadjuvant chemotherapy followed by radical surgery provides the
best survival outcomes [15,17], these conclusions are limited by the
retrospective nature of these reports. A multi-centre randomised
controlled trial will help to validate our results, but we recognize that
trial recruitment would be logistically challenging for such a rare
condition.
This study is the first, as we are aware, to hypothesize a difference
in biological behaviour between pure SCCB and mixed SCCB,
where the presence of urothelial carcinoma in SCCB confers a more
favourable prognosis. The small study size precludes univariate
and multivariate analysis of histological subtype as an important
prognostic factor in survival outcomes. Being aware of the limitations
of a small study sample size, it would be useful to validate this
hypothesis in larger studies. However, the predominant confounders
in survival analysis, such as age, Charlson Comorbidity Index, and
tumour stage at presentation are in favour of the pure SCCB subgroup,
strongly suggesting that histological subtype is a significant
contributor to DSS and OS.
There have been 3 theories regarding the histogenesis of small
cell bladder carcinoma [21]. The first theory is that SCCB is derived
from the Amine Precursor Uptake and Decarboxylation (APUD)
system. APUD cells are neuroendocrine cells located next to the
basal lamina of epithelial surfaces. Although a common cell of origin
is suggested for tumours with mixed SCCB [22], divergent clonality
during tumour progression is hypothesized to lead to different cell
types within the same lesion. This hypothesis provides supporting
evidence, but does not offer a mechanistic explanation for a less
aggressive biological behaviour observed by mixed SCCB [17]. The
second theory is that SCCB is derived from metaplasia of high grade
malignancies, which may explain why SCCB is sometimes found with
other bladder malignancies with a mixed histology [18]. The third
theory is that SCCB stems from multipotential stem cells [18,19] and
may explain why SCCB can exist as a mixed histology or as a pure
histology.
In a study comprising 10 patients, Terraciano et al. [20] used
Comparative Genomic Hybridization (CGH) to analyse copy number
aberrations in the tumours. This study demonstrated that SCCB was
characterised by frequent genomic alterations [23], such as DNA
deletions at 10q, 4q, 5q and 13q, and DNA gains at 8q, 5p, 6p and
20q. In one case with coexistent urothelial carcinoma, both small cell
carcinoma and urothelial carcinoma areas showed similar genotypic
alterations and therefore, a similar clonality.
Therefore, we hypothesize that mixed SCCB may arise from
different genomic alterations distinct from pure SCCB, resulting in
their different clinical behaviour. The elucidation of the mechanistic
pathways behind such a differential phenotype will likely yield
insights into potential therapeutic pathways that may be used in novel
treatment strategies. Although the comparison between pure SCCB
and mixed SCCB was statistically not significant (p=0.09), this was
most likely due to the small sample size of the cohort.
Over the decade from which the study patients were identified, we
recognise that evolution of treatment strategies and patient selection
would contribute biases to outcomes analysis, although its magnitude
of influence is not easily quantifiable. At the same time, we recognise
that limiting the duration of the study period would also lead to a
smaller study population in this rare condition.
While attempts were made to restage all cases using a more
contemporary staging system, the limitations inherent to refinement
of diagnostic imaging modalities in the later part of the study duration
may affect the accuracy of clinical staging. However, given that all
patients underwent axial imaging at diagnosis, it is unlikely that these
changes in imaging technique will affect staging to a large extent. The
strengths of this study include central pathological review and clinical
data obtained from a prospectively maintained cancer registry.
Table 3
Conclusion
Small cell bladder carcinoma is an uncommon disease with an aggressive oncologic behaviour and poor prognosis. Although mixed small cell bladder carcinoma has a relatively better prognosis compared to pure small cell bladder carcinoma, further studies need to be done to prove this observation. Good outcomes are observed in different treatment modalities and large multicentre studies are required to better ascertain best treatment options in view of the rarity of this disease.
References
- Richardson RL, Weiland LH. Undifferentiated small cell carcinomas in extrapulmonary sites. Semin Oncol. 1982; 9: 484-496.
- Ibrahim NB, Briggs JC, Corbishley CM. Extrapulmonary oat cell carcinoma. Cancer. 1984; 54: 1645-1661.
- Remick SC, Ruckdeschel JC. Extrapulmonary and pulmonary small-cell carcinoma: tumour biology, therapy and outcome. Med Pediatr Oncol. 1992; 20: 89-99.
- Mukesh M, Cook N, Hollingdale AE, Ainsworth NL, Russell SG. Small cell carcinoma of the urinary bladder: A 15 year retrospective review of treatment and survival in the Anglian Cancer Network. BJU Int. 2008; 103: 747-752.
- Lo Re G, Canzonieri V, Veronesi A, Dal Bo V, Barzan L, Zancanaro C, et al. Extrapulmonary small cell carcinoma: A single-institution experience and review of the literature. Ann Oncol. 1994; 5: 909-913.
- Grignon DJ, Ro JY, Ayala AG, Shum DT, Ordóñez NG, Logothetis CJ, et al. Small cell carcinoma of the urinary bladder: a clinicopathologic analysis of 22 cases. Cancer. 1992; 69: 527-536.
- Abbas F, Civantos F, Benedetto P, Soloway MS. Small cell carcinoma of the bladder and prostate. Urology. 1995; 46: 617-630.
- Cheng L, Pan CX, Yang XJ, Lopez-Beltran A, MacLennan GT, Lin H, et al. Small cell carcinoma of the urinary bladder: a clinocopathologic analysis of 64 patients. Cancer. 2004; 101: 957-962.
- Lohrisch C, Murray N, Pickles T, Sullivan L. Small cell carcinoma of the bladder; long term outcome with integrated chemoradiation. Cancer. 1999; 86: 2346-2352.
- Choong NW, Quevado JF, Kaur JS. Small cell carcinoma of the urinary bladder. The Mayo Clinic experience. Cancer. 2005; 103: 1172-1178.
- Blomjous CE, Vos W, De Voogt HJ, Van der Valk P, Meijer CJ. Small cell carcinoma of the urinary bladder. A clinicopathologic, morphometric, immunohistochemical, and ultrastructural study of 18 cases. Cancer. 1989; 64: 1347-1357.
- Trias I, Algaba F, Condom E. Small cell carcinoma of the urinary bladder: Presentation of 23 cases and review of 134 published cases. Eur Urol. 2001; 39: 85-90.
- Mackey JR, Au HJ, Hugh J, Venner P. Genitourinary small cell carcinoma: determination of clinical and therapeutic factors associated with survival. J Urol. 1998; 159: 1624-1629.
- Pervez N, El-Gehani F, Joseph K, Dechaphunkul A, Kamal M, Pertschy D, et al. Genitourinary small-cell carcinoma: a single-institution experience Curr Oncol. 2013; 20: 258-264.
- Patel SG, Stimpson CJ, Zaid HB, Resnick MJ, Cookson MS, Barocas DA, et al. Locoregional small cell carcinoma of the bladder: Clinical characteristics and treatment patterns. J Urol. 2014; 191: 329-334.
- Brennan SM, Gregory DL, Stillie A, Herschtal A, Mac Manus M, Ball DL, et al. Should extrapulmonary small cell cancer be managed like small cell lung cancer? Cancer. 2010; 116: 888-895.
- Pease A. The cytochemistry and ultrastructure of polypeptide hormone-producing cells of the APUD series and the embryologic, physiologic and pathologic implications of the concepts. J Histochem Cytochem. 1969; 17: 303-313.
- Christopher ME, Seftel AD, Sorenson K, Resnick M. Small cell carcinoma of the genitourinary tract: an immunohistochemical, electron microscopic and clinicopathological study. J Urol. 1991; 146: 382-388.
- Blomjous CEM, Vos W, DeVoogt HJ. Small cell carcinoma of the urinary bladder. A clinicopathologic, morphologic, immunohistochemical, and ultrastructural study of 18 cases. Cancer.1989; 64: 1347-1357.
- Abbas F, Civantos F, Benedetto P, Soloway MS. Small cell carcinoma of the bladder and prostate. Urology. 1995; 46: 617-630.
- Terracciano L, Richter J, Tornillo L, Beffa L, Diener PA, Maurer R, et al. Chromosomal imbalances in small cell carcinomas of the urinary bladder. J Path. 1999; 189: 230-235.
- Cheng L, Jones TD, McCarthy RP, Eble JN, Wang M, MacLennan GT, et al. Molecular genetic evidence for a common clonal origin of urinary bladder small cell carcinoma and coexisting urothelial carcinoma. Am J Pathol. 2005; 166: 1533-1539.
- Kaushik D, Frank I, Boorjian SA, Cheville JC, Eisenberg MS, Thapa P, et al. Long term results of radical cystectomy and role of adjuvant chemotherapy for small cell carcinoma of the bladder. Int J Urol. 2015; 22: 549-554.