Review Article
Endocrine Disrupting Compounds and Prostate Cell Proliferation
Joubert Banjop Kharlyngdoh and Per-Erik Olsson*
Department of Biology, Örebro University, Sweden
*Corresponding author: Per-Erik Olsson, Department of Biology, The Life Science Center, School of Science and Technology, Örebro University, SE-70182 Örebro, Sweden
Published: 18 Oct, 2016
Cite this article as: Kharlyngdoh JB, Olsson P-E. Endocrine
Disrupting Compounds and Prostate
Cell Proliferation. Clin Oncol. 2016; 1:
1116.
Abstract
Epidemiological, in vitro and animal studies have indicated that Endocrine Disrupting Compounds
(EDCs) influence the normal growth and development of the prostate as well as development and
progression of prostate cancer. This has been linked to an increased presence of environmental
chemicals that interfere with hormonal signaling. Many of these effects appear to be associated with
interferences with steroid hormone receptor signaling or by affecting steroidogenesis. Currently,
there is abundant evidence from epidemiological studies linking pesticides and EDCs with elevated
prostate cancer risk. Bisphenol-A, a known EDC, has been shown to promote induction of Prostate
Specific Antigen (PSA), which is a biomarker for prostate cancer, in the LNCaP human prostate
carcinoma cells, and to increase prostate carcinogenesis in animal models. Our research has focused
on AR signaling and identification of novel androgenic and anti-androgenic EDCs. Using prostate
cancer cell lines we have shown that the AR agonist, TBECH, and antagonist ATE, which is also
a partial ART877A agonist, induce PSA expression. With the increasing presence of these EDCs in
indoor and outdoor air, follows an increased risk of disturbed prostate development, regulation and
function. Hence, identification of the contribution of EDCs to prostate cancer development should
be considered a high priority.
Keywords: Endocrine disrupting compounds; Androgen receptor; Prostate cancer
Abbbreviations
AF: Activating Function; AR: Androgen Receptor; ATE/TBP-AE: Allyl 2, 4, 6-tribromophenyl ether; BATE/TBP-BAE: 2-bromoallyl 2, 4, 6-tribromophenyl ether; DPTE/TBP-DBPE: 2, 3-dibromopropyl-2, 4, 6-tribromophenyl ether; BFR: Brominated Flame Retardant; DBD: DNA Binding Domain; DHT: Dihydrotestosterone; EDC: Endocrine Disrupting Compound; LBD: Ligand Binding Domain; NTD: N-terminal Domain; PCa: Prostate Cancer; PSA: Prostate Specific Antigen; T: Testosterone; TAU: Transcription Activation Unit; TBECH: 1, 2-dibromo-4-(1, 2 dibromoethyl) cyclohexane; AD: Androgen Dependent; AI: Androgen Independent; BPA: Bisphenol A; ER: Estrogen Receptor; PR: Progesterone Receptor; TR: Thyroid Receptor; PPAR: Peroxisome- Proliferator Activated Receptor; PBDE: Polybrominateddiphenyl Ether; H874Y: Histidine 874 Tyrosine, T877A: Threonine 877 Alanine; T877S: Threonine 877 Serine; W741C: Tryphtohan 741 Cysteine; F876L: Phenylalanine 876 Leucine; DHEA: Dehydroepiandrosterone
Androgen Receptor, Mutations and Prostate Cancer
Testosterone (T) and its metabolite 5α-dihydrotestosterone (DHT) are the main male sex
steroids. They are indispensable for male development as well as sexual behavior, and mediate
their biological effects via the Androgen Receptor (AR) [1]. AR is expressed in many organs
such as hypothalamus, liver, pituitary, prostate and testes [2]. AR belongs to the nuclear receptor
subfamily 3, group C, member 4 (NR3C4), a member of the steroid receptor family of ligandinducible
transcription factors [3]. The human AR cDNA was first cloned in 1988 [4], and localized
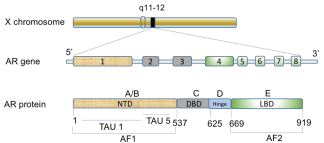
on X chromosome at Xq11-12 [5]. Human AR consists of 8 exons that encode a 110 kDa protein
consisting of 919 amino acids [6]. AR protein has a modular structure, consisting of four structural
domains each harboring an independent function that is crucial for AR action (Figure 1). These are
the NH2-terminal or A/B domain (NTD), the DNA-binding or C domain (DBD), the hinge region
or D domain, and the ligand-binding or E domain (LBD) [7-9].
The NTD, encoded by exon 1 makes up more than half the size of AR (residues 1-555) [6]. The
first 30 residues are highly conserved and crucial for interactions with the LBD, a property that is
unique to AR among the steroid receptor family [10]. The human NTD comprises of polyglutamine
(CAG) and polyglycine (GGC) repeats that is highly variable but
absent in lower organisms like zebrafish [11-13]. In addition to its
variable sequence property, the NTD also contains the transcriptional
activation function AF-1 that is made up of two highly modular
transcriptional units (TAUs) TAU 1 (residues 100-370) and TAU
5 (residues 360-485) [14]. Transcriptional activation of AR occurs
when AF-1 is separated from the LBD. Additionally phosphorylation
of NTD via several intracellular kinases is a well-known posttranslational
modification permitting ligand-independent AR
activation and function [6,10].
The DBD (residues 556-623) is encoded by exons 2 and 3, and is the
most conserved region within the nuclear receptor family. The DBD
region consists of eight cysteine residues forming two zinc fingers,
each made up of four cysteines and a Zn2+ ion that binds to the major
groove of DNA [15]. The first zinc finger contains the proximal box
(P-box) that determines the specificity of DNA sequence recognition
of the AR protein as well as formation of a “recognition helix” [16].
The second zinc finger form the “D-box” (distal box) and is involved
in DNA-dependent AR dimerization [16]. AR binds to target
androgen response elements (AREs) in a head-to-head dimer-like
manner [17]. Apart from DNA binding, the DBD also play a vital role
in mediating nuclear localization and dimerization of AR. The hinge
region or D domain serves as a flexible linker connecting DBD and
LBD, and also contains the nuclear localization signal that influences
AR subcellular location. The hinge region is also involved in DNA
binding, coactivator recruitment and AR dimerization [18,19].
The AR LBD (666-919) is a well characterized structure and
serves primarily to bind androgens [20]. The AR LBD is made up
of only 11 α-helices, rather than 12, due to the absence of helix 2,
found in other nuclear receptors. It also contain four short β strands
forming two anti-parallel β-sheets [7,20-22].The AR LBD is identical
for humans, rats and mice and offers high affinity binding of the two
endogenous androgens, T and DHT [23]. Like other steroid receptors,
the LBD contains a transcriptional activation function 2 (AF-2)
that is ligand dependent. The AF-2 co-activator surface serves to
recruit co-activators such as p160 thereby promoting transcriptional
activity [22]. In contrast, binding of AR antagonists do not induce
a similar repositioning of helix-12 thereby leading to recruitment of
co-repressors such as the nuclear receptor co-repressor (NCoR) and
the silencing mediator of retinoic acid and thyroid hormone receptor
(SMRT) [24].
The prostate depends on circulating androgens for normal growth,
development and function [25]. In rodents and humans, the loss of
AR is associated with failure of prostate development [26,27]. The
development and metastasis of prostate cancer (PCa) is dependent
on AR activation by androgens. Globally, PCa is the second most
frequently diagnosed cancer in men [28]. Treatment of metastatic
PCa involves either androgen ablation monotherapy or antiandrogen
drug treatment. AR positive PCa tumors can either display
androgen-dependent or androgen-independent characteristics.
Occasionally PCa can show heterogeneity within a tumor, with the
presence of both androgen-dependent and androgen-independent
cells [29,30]. Initially most PCa tumors are dependent on circulating
androgens for growth, and treatment is therefore aimed at lowering
serum androgen levels [31]. In some patients it has been observed
that the anti-androgens convert to AR agonists when PCa has
reached advanced stages [32]. AR is prone to mutations and so far 159
mutations have been detected in PCa tissue with a majority of them
being single base substitutions [33]. Among these 45% are present
within the AR-LBD. The most frequently detected substitution
mutation in PCa tumors is that of codon 877 which encode threonine
and is substituted by alanine (ART877A). This mutation comprises
25-31% of the mutations in advanced PCa patients treated with
androgen ablation therapy [34,35]. The presence of ART877A mutations
within the LBD render AR non-specifically activated by different
ligands that include dehydroepiandrosterone (DHEA), estrogens,
phytoestrogens, progestogens, anti-androgens such as cyproterone
acetate, hydroxyflutamide and nilutamide [36,37]. Another PCa
associated mutation within the codon 877 where threonineis
substituted by serine (ART877S) exhibited a potent activation by
estradiol, progesterone and cyproterone acetate [38]. Another
mutation, ARH874Y showed a similar response as ART877A to a diverse
group of ligands [36,38]. The anti-androgenic drug bicalutamide
exhibited agonist activity to AR with the W741C mutation within the
LBD that is detected in advanced PCa patients [39,40]. Recent studies
have also shown that the mutation ARF876L within the LBD convert the
second-generation PCa drug enzalutamide into an agonist [41-43].
Figure 1
Figure 1
Structural and functional organization of the human AR gene
and protein. A schematic mapping of the AR gene on the long arm of the
X-chromosome, displaying the eight exons that codes for a 919 amino acids
long protein made up of different functional domains.
Abbreviations: NTD: NH2-Terminal Domain; DBD: DNA Binding Domain;
LBD: Ligand Binding Domain; AF: Activation Function; TAU: Transcriptional
Activation Unit
Exon 1 encodes for the NTD, exons 2 and 3 encode the DBD, and exons 4 to
8 encode both the hinge and LBD. Adapted from Lonergan PE, Tindall, DJ.
J Carcinog. 2011; 10: 20.
Endocrine Disrupting Compounds
According to the Endocrine Society, an EDC is defined as “an
exogenous chemical, or mixture of chemicals, that interferes with
any aspect of hormone action” [44]. EDCs interact with receptors by
mimicking hormones and exert effects on target cells via activation or
repression of target genes. An example of this is bisphenol A (BPA),
an estrogen mimic that binds to the Estrogen Receptor (ER) [45,46].
Originally EDCs were thought to be mediating their effects mainly
through nuclear receptors such as the ER, AR, Progesterone Receptor
(PR), Thyroid Receptor (TR), and retinoid receptor [47]. However,
it is now understood that the EDC mechanisms of action are more
diverse than originally believed. Apart from steroid nuclear receptors,
EDCs exert their effects through other nuclear receptors such as the
Peroxisome Proliferator Activated Receptor (PPAR). Phthalates
have been shown to exert adverse effects on reproductive functions
through exposure of direct activation of PPARs [48,49]. EDCs have
also been reported to mediate their actions via neurotransmitter
receptors, aryl hydrocarbon receptors, different enzymes involved in
steroid metabolism, and other mechanisms related with endocrine
regulation and reproduction [47,50].
BPA which is a well-known ER agonist, is also a TR antagonist
and an AR wild type antagonist and an agonist to ART877A [51,52].
EDCs can also exert their effects directly or through metabolic
products like OH/Me OH polybrominateddiphenyl ethers (PBDEs),
polychlorobiphenylols, dichlorodiphenyldichloroethylene and
dichlorodiphenyl-dichloroethane [53-56]. Apart from interfering
with hormonal signaling pathways, recent reports have also shown
that EDCs can induce epigenetic changes in target tissues, for instance
alteration in DNA methylation patterns in prostate and testicular
cells following exposure to BPA, the antiandrogen vinclozolin and
diethylhexyl phthalate [57-59].
Androgenic and Anti-Androgenic Compounds
There are a large number of compounds that have either been
discovered or synthesized to interact with AR [60-63]. AR mediated
EDCs have been identified via in silico approach as well as in vitro
experiments. Hence, on the basis of their ability to activate or repress
AR transcriptional activity, EDCs can be grouped into androgenic/
agonist and anti-androgenic/antagonist compounds. Naturally
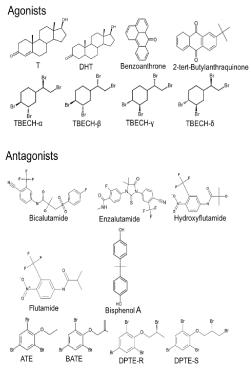
occurring androgens consist of T and its metabolite DHT (Figure 2).
AR agonists mimic endogenous androgens and trigger androgenic
responses, while AR antagonist repress AR transactivation. Some of
the well-known anti-androgenic compounds are steroidal in nature
such as chlormadinoneacetate, cyproterone acetate and allylestrenol,
while others are non-steroidal like bicalutamide, flutamide,
hydroxyflutamide and enzalutamide (Figure 2) [64,65]. There are
numerous studies where in silico screening or reporter assays or both
has been used to identify compounds with AR binding activity. A
majority of the reported compounds exhibit anti-androgenic activity
while only a few show androgenic activity [60,62,63,66-73]. From
the systematic screening of different compounds, the first reported
case of environmental chemicals exhibiting AR agonistic properties
were 2-tert-butylanthraquinone and benzoanthrone (Figure 2),
which were relatively weak partial agonists with only 10% maximal
activation relative to the natural ligand DHT [62].
The first potent environmental AR agonist was 1,2-dibromo-4-(1,2
dibromoethyl) cyclohexane (TBECH/DBE-DBCH) [74], and TBECH
(Figure 2) was also later shown to act as AR agonist for chicken and
zebrafish AR using in silico, in vitro, and in vivo approaches [75,76].
2-tert-Butylanthraquinone and Benzanthrone are fused polycyclic
hydrocarbons and were identified as AR agonists from a screen of
253 industrial chemicals in a study using the AR EcoScreen assay
[62]. However, these agonists displayed weak responses, being
approximately 1000-10,000 fold less potent than DHT. TBECH
is a Brominated Flame Retardant (BFR) used as an additive in
electrical appliances, plastics, fabric adhesives, and in polystyrene
and polyurethane [77]. It exist as four diastereomers (α, β, γ and δ)
each with its own enantiomer [77]. Due to the potency of TBECH in
activating AR at nanomolar concentrations, it has been ranked as one
of the 10% most hazardous compounds to ecosystems [78]. TBECH
has been identified in sediments, living organisms, and in indoor
and outdoor air [79]. TBECH is commercially available as a mixture
of α:β in the marketed flame retardant Saytex BCL 462 (Albemarle
Corporation), but at temperature >120°C the α and β forms are
converted to γ and δ [77]. Among the four diastereomers, γ and δ are
more potent at activating AR across species [75,76,80]. TBECH has
been shown to be maternally transferred and cause delayed hatching
in zebrafish [76,81]. TBECH has also been shown to be mutagenic
in the L5178Y tk+/tk- mouse lymphoma-cell forward-mutation assay
[82].
While few androgen agonists have been identified, there are
more reports on environmental and industrial compounds exhibiting
anti-androgenic properties. The most common AR antagonists are
agricultural products such as pesticides, herbicides, insecticides, and
fungicides. AR antagonists have also been identified from products
used in plastics and other industrial products.
PBDEs and their congeners, are brominated flame retardants
used in commercial products such as electronic equipment and
textiles [83]. The PBDE congeners DE-71, BDE-47 and BDE-100
have been identified as AR antagonists through in vitro and in vivo
analyses. Exposure to these PBDEs resulted in decreased size of male
accessory genital glands [84]. In a study on male Wistar rats, exposure
to DE-71 resulted in delayed puberty as well as decreased size of the
prostate and seminal vesicle [85].
BPA is a synthetic polymer widely used for manufacturing of
polycarbonate plastics and epoxy resins [86]. While BPA primarily
act as an ER agonist, recent studies have shown that it binds to AR
and display AR antagonistic activity [51]. A study on rodents have
shown that it reduces sperm count [87] and it has also been linked to
erectile dysfunction in men having high levels of BPA in their urine
[88]. Studies from rodent models and human PCa cell lines indicate
that BPA is carcinogenic and stimulate tumors progression [89-91].
BPA activate the mutated ART877A frequently found in PCa patients
who relapse following androgen ablation therapy [52]. This indicates
that further studies are needed to better understand the role of BPA
in PCa progression.
Allyl 2, 4, 6-tribromophenyl ether (ATE/TBP-AE),
2-bromoallyl 2, 4, 6-tribromophenyl ether (BATE/TBP-BAE) and 2,
3-dibromopropyl-2, 4, 6-tribromophenyl ether (DPTE/TBP-DBPE)
(Figure 2) are a novel group of BFRs that we recently identified as
AR antagonists [92]. These compounds have been detected in the
environment, house dust and in aquatic animals. Among these, only
ATE and DPTE, that exist in two iso-forms DPTE-R and DPTE-S,
were used as flame retardants, whereas BATE is a by-product of
DPTE biotransformation and has never been used as a BFR [93]. ATE
is the main constituent of the BFR PHE-65 (Great Lakes Chemical
Corporation) and is currently still in use, while DPTE was the main
constituent of the BFR Bromkal 73-5PE (Chemische Fabrik Kalk)
until the mid 1980s [94,95]. These AR antagonists are equally potent
at inhibiting AR transcriptional activity across different species,
including human, chicken and zebrafish [92,96,97].
Figure 2
Figure 2
Molecular structures of androgens and EDCs that interact with
AR. Agonists: T, DHT, Benzoanthrne, 2-tert-Butylanthraquinone, TBECH
isoforms α, β, γ and δ; and Antagonists: Bicalutamide, Enzalutamide,
Hydroxyfutamide, Flutamide, Bisphenol A, ATE, BATE, and DPTE-R and –S
isoforms.
TBECH and Androgen Receptor Mutations
TBECH is present in the commercially available product Saytex BCL 462 as an equimolar mixture of TBECH α and β [77]. We have shown that all four TBECH diastereomers induce expression of prostate specific antigen (PSA) in human prostate carcinoma LNCaP cells harboring the ART877A mutation [80]. The TBECH diastereomers were more potent at transcriptionally activating the mutated ARW741C and ART877A than the wild type AR [98]. TBECH γ and δ are much more potent than α and β at activating AR [80]. While TBECH γ and δ are as potent as DHT, only high concentrations of α:β and β (1 μM and 10 μM) are able to induce transcriptional activation of ARW741C. The α:β and β iso-forms are also more potent at activating ART877A when compared to ARW741C and ARWT. Both α:β and γ:δ induced PSA expression to comparable levels in the human LNCaP cell line. Although currently no epidemiological studies have reported a link between the TBECH and PCa progression, the mechanistic results indicate that TBECH could interfere with PCa progression.
ATE/DPTE and Prostate Cancer Drugs, Comparison of Potency
Currently only ATE is in use as a BFR, as the production of DPTE ceased in the mid 1980s due to a fire incidence in the factory where it was manufactured [94,95]. The two BFRs were equally potent at inhibiting DHT-induced AR transcriptional activity in HeLa cells [92], while DPTE is more potent at inhibiting DHT-induced PSA expression in LNCaP cell lines when compared to ATE. Interestingly, when LNCaP cells were exposed to ATE alone there was a low level induction of PSA expression, indicating that ATE acts as a partial agonist to ART877A present in LNCaP cells. Co-exposure of HeLa cells to DHT with DPTE or either of the three PCa drugs bicalutamide, flutamide and hydroxyflutamide, resulted in comparable dose dependent inhibition of DHT-induced AR transcriptional activity and PSA expression. This indicates that DPTE is equally potent as the PCa drugs at inhibiting AR activity [92]. Exposure of LNCaP cells harboring the ART877A mutation to hydroxyflutamide resulted in induction of PSA expression. This showed that both ATE and hydroxyflutamide are partial agonists to LNCaP ART877A, suggesting that exposure of PCa patients harboring this mutation could lead to PCa progression.
Conclusion on Possible Involvement of EDC in Prostate Cancer Progression
TBECH, apart from activating AR, exhibit mutagenic activity, thereby suggesting that it may act as a carcinogen. In addition, as the AR antagonist ATE exhibit partial agonistic property towards ART877A it may also be an EDC capable of stimulating PCa growth and progression. In order to establish a direct link between EDCs and PCa, epidemiological determination of correlations between exposure and health are clearly needed.
Acknowledgments
The research was financed by the Knowledge Foundation and Örebro University, Sweden.
References
- Li J, Al-Azzawi F. Mechanism of androgen receptor action. Maturitas. 2009; 63: 142-148.
- Matsumoto T, Shiina H, Kawano H, Sato T, Kato S. Androgen receptor functions in male and female physiology. J Steroid Biochem Mol Biol. 2008; 109: 236-241.
- Nuclear Receptors Nomenclature Committee. A unified nomenclature system for the nuclear receptor superfamily. Cell. 1999; 97: 161-163.
- Lubahn DB, Joseph DR, Sullivan PM, Willard HF, French FS, Wilson EM. Cloning of human androgen receptor complementary DNA and localization to the X chromosome. Science. 1988; 240: 327-330.
- Brown CJ, Goss SJ, Lubahn DB, Joseph DR, Wilson EM, French FS. Androgen receptor locus on the human X chromosome: regional localization to Xq11-12 and description of a DNA polymorphism. Am J Hum Genet. 1989; 44: 264-269.
- Gelmann EP. Molecular biology of the androgen receptor. J Clin Oncol. 2002; 20: 3001-3015.
- Dehm SM, Tindall DJ. Androgen receptor structural and functional elements: role and regulation in prostate cancer. Mol Endocrinol. 2007; 21: 2855-2863.
- Tsai MJ, O'Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994; 63: 451-486.
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, et al. The nuclear receptor superfamily: the second decade. Cell. 1995; 83: 835-839.
- McEwan IJ. Molecular mechanisms of androgen receptor-mediated gene regulation: structure-function analysis of the AF-1 domain. Endocr Relat Cancer. 2004; 11: 281-293.
- Hsing AW, Gao YT, Wu G, Wang X, Deng J, Chen YL, et al. Polymorphic CAG and GGN repeat lengths in the androgen receptor gene and prostate cancer risk: a population-based case-control study in China. Cancer Res. 2000; 60: 5111-5116.
- Sasaki M, Kaneuchi M, Sakuragi N, Fujimoto S, Carroll PR, Dahiya R. The polyglycine and polyglutamine repeats in the androgen receptor gene in Japanese and Caucasian populations. Biochem Biophys Res Commun. 2003; 312: 1244-1247.
- Hossain MS, Larsson A, Scherbak N, Olsson PE, Orban L. Zebrafish androgen receptor: isolation, molecular, and biochemical characterization. Biol Reprod. 2008; 78: 361-369.
- Jenster G, van der Korput HA, Trapman J, Brinkmann AO. Identification of two transcription activation units in the N-terminal domain of the human androgen receptor. J Biol Chem. 1995; 270: 7341-7346.
- Härd T, Kellenbach E, Boelens R, Maler BA, Dahlman K, Freedman LP. Solution structure of the glucocorticoid receptor DNA-binding domain. Science. 1990; 249: 157-160.
- Laudet V, Gronemeyer H. The Nuclear Receptor Factsbook. Academic Press. 2002. San Diego.
- Shaffer PL, Jivan A, Dollins DE, Claessens F, Gewirth DT. Structural basis of androgen receptor binding to selective androgen response elements. Proc Natl Acad Sci U S A. 2004; 101: 4758-4763.
- Clinckemalie L, Vanderschueren D, Boonen S, Claessens F. The hinge region in androgen receptor control. Mol Cell Endocrinol, 2012; 358: 1-8.
- Haelens A, Tanner T, Denayer S, Callewaert L, Claessens F. The hinge region regulates DNA binding, nuclear translocation, and transactivation of the androgen receptor. Cancer Res. 2007; 67: 4514-4523.
- Matias PM, Donner P, Coelho R, Thomaz M, Peixoto C, Macedo S. et al. Structural evidence for ligand specificity in the binding domain of the human androgen receptor. Implications for pathogenic gene mutations. J Biol Chem. 2000; 275: 26164-26171.
- Moras D; Gronemeyer H. The nuclear receptor ligand-binding domain: structure and function. Curr Opin Cell Biol. 1998; 10: 384-391.
- Sack JS, Kish KF, Wang C, Attar RM, Kiefer SE, An Y. Crystallographic structures of the ligand-binding domains of the androgen receptor and its T877A mutant complexed with the natural agonist dihydrotestosterone. Proc Natl Acad Sci U S A. 2001; 98: 4904-4909.
- Hiipakka RA, Liao S. Molecular mechanism of androgen action. Trends Endocrinol Metab. 1998; 9: 317-324.
- Hodgson MC, Shen HC, Hollenberg AN, Balk SP. Structural basis for nuclear receptor corepressor recruitment by antagonist-liganded androgen receptor. Mol Cancer Ther. 2008; 7: 3187-3194.
- Cunha GR, Donjacour AA, Cooke PS, Mee S, Bigsby RM, Higgins SJ, et al. The endocrinology and developmental biology of the prostate. Endocr Rev. 1987; 8: 338-362.
- WW He, M V Kumar, Tindall DJ. A frame-shift mutation in the androgen receptor gene causes complete androgen insensitivity in the testicular-feminized mouse. Nucleic Acids Res. 1991; 19: 2373-2378.
- T R Brown, D B Lubahn, E M Wilson, D R Joseph, F S French, C J Migeon, et al. Deletion of the steroid-binding domain of the human androgen receptor gene in one family with complete androgen insensitivity syndrome: evidence for further genetic heterogeneity in this syndrome. Proc Natl Acad Sci U S A. 1988; 85: 8151-8155.
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65: 87-108.
- Isaacs JT, Coffey DS. Adaptation versus selection as the mechanism responsible for the relapse of prostatic cancer to androgen ablation therapy as studied in the Dunning R-3327-H adenocarcinoma. Cancer Res. 1981; 41: 5070-5075.
- Isaacs JT, Lundmo PI, Berges R, Martikainen P, Kyprianou N, English HF. Androgen regulation of programmed death of normal and malignant prostatic cells. J Androl. 1992; 13: 457-464.
- Katzenwadel A, Wolf P. Androgen deprivation of prostate cancer: Leading to a therapeutic dead end. Cancer Lett. 2015; 367: 12-17.
- Ahmed A, Ali S, Sarkar FH. Advances in androgen receptor targeted therapy for prostate cancer. J Cell Physiol. 2014; 229: 271-276.
- Gottlieb B, Beitel LK, Nadarajah A, Paliouras M, Trifiro M. The androgen receptor gene mutations database: 2012 update. Hum Mutat. 2012; 33: 887-894.
- Gaddipati JP, McLeod DG, Heidenberg HB, Sesterhenn IA, Finger MJ, Moul JW, et al. Frequent detection of codon 877 mutation in the androgen receptor gene in advanced prostate cancers. Cancer Res. 1994; 54: 2861-2864.
- Taplin ME, Bubley GJ, Ko YJ, Small EJ, Upton M, Rajeshkumar B, et al. Selection for androgen receptor mutations in prostate cancers treated with androgen antagonist. Cancer Res. 1999; 59: 2511-2515.
- Tan J, Sharief Y, Hamil KG, Gregory CW, Zang DY, Sar M, et al. Dehydroepiandrosterone activates mutant androgen receptors expressed in the androgen-dependent human prostate cancer xenograft CWR22 and LNCaP cells. Mol Endocrinol. 1997; 11: 450-459.
- Veldscholte J, Voorhorst-Ogink MM, Bolt-de Vries J, van Rooij HC, Trapman J, Mulder E. Unusual specificity of the androgen receptor in the human prostate tumor cell line LNCaP: high affinity for progestagenic and estrogenic steroids. Biochim Biophys Acta. 1990; 1052: 187-194.
- Steketee K, Timmerman L, Ziel-van der Made AC, Doesburg P, Brinkmann AO, Trapman J, et al. Broadened ligand responsiveness of androgen receptor mutants obtained by random amino acid substitution of H874 and mutation hot spot T877 in prostate cancer. Int J Cancer. 2002; 100: 309-317.
- Hara T, Miyazaki J, Araki H, Yamaoka M, Kanzaki N, Kusaka M, et al. Novel mutations of androgen receptor: a possible mechanism of bicalutamide withdrawal syndrome. Cancer Res. 2003; 63: 149-153.
- Taplin ME, Rajeshkumar B, Halabi S, Werner CP, Woda BA, Picus J, et al. Androgen receptor mutations in androgen-independent prostate cancer: Cancer and Leukemia Group B Study 9663. J Clin Oncol. 2003; 21: 2673-2678.
- Balbas MD, Evans MJ, Hosfield DJ, Wongvipat J, Arora VK, Watson PA, et al. Overcoming mutation-based resistance to antiandrogens with rational drug design. Elife. 2013; 2: e00499.
- Joseph JD, Lu N, Qian J, Sensintaffar J, Shao G, Brigham D, et al. A clinically relevant androgen receptor mutation confers resistance to second-generation antiandrogens enzalutamide and ARN-509. Cancer Discov. 2013; 3: 1020-1029.
- Korpal M, Korn JM, Gao X, Rakiec DP, Ruddy DA, Doshi S, et al. An F876L mutation in androgen receptor confers genetic and phenotypic resistance to MDV3100 (enzalutamide). Cancer Discov. 2013; 3: 1030-1043.
- Zoeller RT, Brown TR, Doan LL, Gore AC, Skakkebaek NE, Soto AM, et al. Endocrine-disrupting chemicals and public health protection: a statement of principles from The Endocrine Society. Endocrinology. 2012; 153: 4097-4110.
- Aranda A, Pascual A. Nuclear hormone receptors and gene expression. Physiol Rev. 2001; 81: 1269-1304.
- Rouiller-Fabre V, Guerquin MJ, N'Tumba-Byn T, Muczynski V, Moison D, Tourpin S, et al. Nuclear receptors and endocrine disruptors in fetal and neonatal testes: a gapped landscape. Front Endocrinol (Lausanne). 2015; 6: 58.
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009; 30: 293-342.
- Rouiller-Fabre V, Habert R, Livera G. Effects of endocrine disruptors on the human fetal testis. Ann Endocrinol (Paris). 2014; 75: 54-57.
- Latini G, Scoditti E, Verrotti A, De Felice C, Massaro M. Peroxisome proliferator-activated receptors as mediators of phthalate-induced effects in the male and female reproductive tract: epidemiological and experimental evidence. PPAR Res. 2008; 2008: 359267.
- T M Crisp, E D Clegg, R L Cooper, W P Wood, D G Anderson, K P Baetcke, et al. Environmental endocrine disruption: an effects assessment and analysis. Environ Health Perspect. 1998; 106: 11-56.
- Lee HJ, Chattopadhyay S, Gong EY, Ahn RS, Lee K. Antiandrogenic effects of bisphenol A and nonylphenol on the function of androgen receptor. Toxicol Sci. 2003; 75: 40-46.
- Wetherill YB, Fisher NL, Staubach A, Danielsen M, de Vere White RW, Knudsen KE. Xenoestrogen action in prostate cancer: pleiotropic effects dependent on androgen receptor status. Cancer Res. 2005; 65: 54-65.
- Metcalf RL, Kapoor IP, Lu PY, Schuth CK, Sherman P. Model ecosystem studies of the environmental fate of six organochlorine pesticides. Environ Health Perspect. 1973; 4: 35-44.
- Hoekstra PF, Letcher RJ, O'Hara TM, Backus SM, Solomon KR, Muir DC. Hydroxylated and methylsulfone-containing metabolites of polychlorinated biphenyls in the plasma and blubber of bowhead whales (Balaena mysticetus). Environ Toxicol Chem. 2003; 22: 2650-2658.
- Orn U, Klasson-Wehler E. Metabolism of 2,2',4,4'-tetrabromodiphenyl ether in rat and mouse. Xenobiotica. 1998; 28: 199-211.
- Wang X, Yang H, Hu X, Zhang X, Zhang Q, Jiang H, et al. Effects of HO-/MeO-PBDEs on androgen receptor: in vitro investigation and helix 12-involved MD simulation. Environ Sci Technol. 2013; 47: 11802-11809.
- Prins GS, Tang WY, Belmonte J, Ho SM. Perinatal exposure to oestradiol and bisphenol A alters the prostate epigenome and increases susceptibility to carcinogenesis. Basic Clin Pharmacol Toxicol. 2008; 102: 134-138.
- Guerrero-Bosagna C, Settles M, Lucker B, Skinner MK. Epigenetic transgenerational actions of vinclozolin on promoter regions of the sperm epigenome. PLoS One. 2010; 5.
- Wu S, Zhu J, Li Y, Lin T, Gan L, Yuan X, et al. Dynamic epigenetic changes involved in testicular toxicity induced by di-2-(ethylhexyl) phthalate in mice. Basic Clin Pharmacol Toxicol. 2010; 106: 118-123.
- Fang H, Tong W, Branham WS, Moland CL, Dial SL, Hong H, et al. Study of 202 natural, synthetic, and environmental chemicals for binding to the androgen receptor. Chem Res Toxicol. 2003; 16: 1338-1358.
- Singh SM, Gauthier S, Labrie F. Androgen receptor antagonists (antiandrogens): structure-activity relationships. Curr Med Chem. 2000; 7: 211-247.
- Araki N, Ohno K, Nakai M, Takeyoshi M, Iida M. Screening for androgen receptor activities in 253 industrial chemicals by in vitro reporter gene assays using AR-EcoScreen cells. Toxicol in vitro. 2005; 19: 831-842.
- Araki N, Ohno K, Takeyoshi M, Iida M. Evaluation of a rapid in vitro androgen receptor transcriptional activation assay using AR-EcoScreen cells. Toxicol in vitro. 2005; 19: 335-352.
- Merseburger AS, Haas GP, von Klot CA. An update on enzalutamide in the treatment of prostate cancer. Ther Adv Urol. 2015; 7: 9-21.
- Urushibara M, Ishioka J, Hyochi N, Kihara K, Hara S, Singh P, et al. Effects of steroidal and non-steroidal antiandrogens on wild-type and mutant androgen receptors. Prostate. 2007. 67: 799-807.
- Hong H, Fang H, Xie Q, Perkins R, Sheehan DM, Tong W. Comparative molecular field analysis (CoMFA) model using a large diverse set of natural, synthetic and environmental chemicals for binding to the androgen receptor. SAR QSAR Environ Res. 2003; 14: 373-388.
- Kojima H, Takeuchi S, Van den Eede N, Covaci A. Effects of primary metabolites of organophosphate flame retardants on transcriptional activity via human nuclear receptors. Toxicol Lett. 2016; 245: 31-39.
- Kojima H, Takeuchi S, Itoh T, Iida M, Kobayashi S, Yoshida T. In vitro endocrine disruption potential of organophosphate flame retardants via human nuclear receptors. Toxicology. 2013; 314: 76-83.
- Takeuchi S, Shiraishi F, Kitamura S, Kuroki H, Jin K, Kojima H. Characterization of steroid hormone receptor activities in 100 hydroxylated polychlorinated biphenyls, including congeners identified in humans. Toxicology. 2011; 289: 112-121.
- Kojima H, Takeuchi S, Uramaru N, Sugihara K, Yoshida T, Kitamura S. Nuclear hormone receptor activity of polybrominated diphenyl ethers and their hydroxylated and methoxylated metabolites in transactivation assays using Chinese hamster ovary cells. Environ Health Perspect. 2009; 117: 1210-1218.
- Liu H, Hu W, Sun H, Shen O, Wang X, Lam MH, et al. In vitro profiling of endocrine disrupting potency of 2,2',4,4'-tetrabromodiphenyl ether (BDE47) and related hydroxylated analogs (HO-PBDEs). Mar Pollut Bull. 2011; 63: 287-296.
- Svobodová K, Placková M, Novotná V, Cajthaml T. Estrogenic and androgenic activity of PCBs, their chlorinated metabolites and other endocrine disruptors estimated with two in vitro yeast assays. Sci Total Environ. 2009; 407: 5921-5925.
- Wu B, Ford T, Gu JD, Zhang XX, Li AM, Cheng SP. Computational studies of interactions between endocrine disrupting chemicals and androgen receptor of different vertebrate species. Chemosphere. 2010; 80: 535-541.
- Larsson A, Eriksson LA, Andersson PL, Ivarson P, Olsson PE. Identification of the brominated flame retardant 1,2-dibromo-4-(1,2-dibromoethyl)cyclohexane as an androgen agonist. J Med Chem. 2006; 49: 7366-7372.
- Asnake S, Pradhan A, Banjop-Kharlyngdoh J, Modig C, Olsson PE. 1,2-Dibromo-4-(1,2 dibromoethyl) cyclohexane (TBECH)-mediated steroid hormone receptor activation and gene regulation in chicken LMH cells. Environ Toxicol Chem. 2014; 33: 891-899.
- Pradhan A, Kharlyngdoh JB, Asnake S, Olsson PE. The brominated flame retardant TBECH activates the zebrafish (Danio rerio) androgen receptor, alters gene transcription and causes developmental disturbances. Aquat Toxicol. 2013; 142-143: 63-72.
- Arsenault G, Lough A, Marvin C, McAlees A, McCrindle R, MacInnis G, et al. Structure characterization and thermal stabilities of the isomers of the brominated flame retardant 1,2-dibromo-4-(1,2-dibromoethyl)cyclohexane. Chemosphere. 2008; 72: 1163-1170.
- EPA, U.S., Waste Minimization Prioritization Tool: Background Document for the Tier PBT Chemical List. Appendix A: WMPT Summary Spreadsheet, U.S.E.P. Agency, Washington, DC, 2000.
- Newton S, Sellstrom U, de Wit CA. Emerging flame retardants, PBDEs, and HBCDDs in indoor and outdoor media in Stockholm, Sweden. Environ Sci Technol. 2015; 49: 2912-2920.
- Khalaf H, Larsson A, Berg H, McCrindle R, Arsenault G, Olsson PE. Diastereomers of the brominated flame retardant 1,2-dibromo-4-(1,2 dibromoethyl)cyclohexane induce androgen receptor activation in the hepg2 hepatocellular carcinoma cell line and the lncap prostate cancer cell line. Environ Health Perspect. 2009; 117: 1853-1859.
- Nyholm JR, Norman A, Norrgren L, Haglund P, Andersson PL. Maternal transfer of brominated flame retardants in zebrafish (Danio rerio). Chemosphere. 2008; 73: 203-208.
- McGregor DB, Brown AG, Howgate S, McBride D, Riach C, Caspary WJ. Responses of the L5178Y mouse Lymphoma cell forward mutation assay. V: 27 coded chemicals. Environ Mol Mutagen. 1991; 17: 196-219.
- Birnbaum LS, Staskal DF. Brominated flame retardants: cause for concern? Environ Health Perspect. 2004; 112: 9-17.
- Stoker TE, Cooper RL, Lambright CS, Wilson VS, Furr J, Gray LE. In vivo and in vitro anti-androgenic effects of DE-71, a commercial polybrominated diphenyl ether (PBDE) mixture. Toxicol Appl Pharmacol. 2005; 207: 78-88.
- Stoker TE, Laws SC, Crofton KM, Hedge JM, Ferrell JM, Cooper RL, et al. Assessment of DE-71, a commercial polybrominated diphenyl ether (PBDE) mixture, in the EDSP male and female pubertal protocols. Toxicol Sci. 2004; 78: 144-155.
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL, et al. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ Health Perspect. 2008; 116: 39-44.
- Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, et al. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. 2007; 24: 199-224.
- Li D1, Zhou Z, Qing D, He Y, Wu T, Miao M, et al. Occupational exposure to bisphenol-A (BPA) and the risk of self-reported male sexual dysfunction. Hum Reprod. 2010; 25: 519-527.
- Ho SM, Tang WY, Belmonte de Frausto J, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006; 66: 5624-5632.
- Wetherill YB, Hess-Wilson JK, Comstock CE, Shah SA, Buncher CR, Sallans L, et al. Bisphenol A facilitates bypass of androgen ablation therapy in prostate cancer. Mol Cancer Ther. 2006; 5: 3181-3190.
- Prins GS, Hu WY, Shi GB, Hu DP, Majumdar S, Li G, et al. Bisphenol A promotes human prostate stem-progenitor cell self-renewal and increases in vivo carcinogenesis in human prostate epithelium. Endocrinology. 2014; 155: 805-817.
- Kharlyngdoh JB, Pradhan A, Asnake S, Walstad A, Ivarsson P, Olsson PE. Identification of a group of brominated flame retardants as novel androgen receptor antagonists and potential neuronal and endocrine disrupters. Environ Int. 2015; 74: 60-70.
- von der Recke R, Vetter W. Synthesis and characterization of 2,3-dibromopropyl-2,4,6-tribromophenyl ether (DPTE) and structurally related compounds evidenced in seal blubber and brain. Environ Sci Technol. 2007; 41: 1590-1595.
- Vetter W, Recke Rv, Ostrowicz P, Rosenfelder N. Liquid chromatographic enantioseparation of the brominated flame retardant 2,3-dibromopropyl-2,4,6-tribromophenyl ether (DPTE) and enantiomer fractions in seal blubber. Chemosphere. 2010; 78: 134-138.
- Covaci A, Harrad S, Abdallah MA, Ali N, Law RJ, Herzke D, et al. Novel brominated flame retardants: a review of their analysis, environmental fate and behaviour. Environ Int. 2011; 37: 532-556.
- Pradhan A, Asnake S, Kharlyngdoh JB, Modig C, Olsson PE. In silico and biological analysis of anti-androgen activity of the brominated flame retardants ATE, BATE and DPTE in zebrafish. Chem Biol Interact. 2015; 233: 35-45.
- Asnake S, Pradhan A, Kharlyngdoh JB, Modig C, Olsson PE. The brominated flame retardants TBP-AE and TBP-DBPE antagonize the chicken androgen receptor and act as potential endocrine disrupters in chicken LMH cells. Toxicol In Vitro. 2015; 29: 1993-2000.
- Kharlyngdoh JB, Asnake S, Pradhan A, Olsson PE. TBECH, 1,2-dibromo-4-(1,2 dibromoethyl) cyclohexane, alters androgen receptor regulation in response to mutations associated with prostate cancer. Toxicol Appl Pharmacol. 2016; 307: 91-101.