Case Report
Appendix Insulin Secreting Neuroendocrine Tumor in a Diabetic Patient: A Challenging Diagnosis
Lombardi M1*, Battezzati MA2, Grosso F3#, Muni A4, Volante M5# and Ansaldi E2
1Department of Internal Medicine, St Antonio Abate Hospital, Italy
2Department of Endocrionology, SS. Antonio and Biagio and Cesare Arrigo Hospital, Italy
3Department of Oncology, SS. Antonio and Biagio and Cesare Arrigo Hospital, Italy
4Department of Nuclear Medicine, SS. Antonio and Biagio and Cesare Arrigo Hospital, Italy
5Department of Oncology, Pathology Unit, University of Turin, Italy
#Italian Rare Cancer Network
*Corresponding author: Martina Lombardi, Department of Internal Medicine, St Antonio Abate Hospital, Pontremoli, USL Toscana Nordovest, Via Nazionale 16, 54027, Pontremoli, Massa Carrara, Italy
Published: 28 Sep, 2016
Cite this article as: Lombardi M, Battezzati MA, Grosso F, Muni A, Volante M, Ansaldi E. Appendix Insulin Secreting Neuroendocrine Tumor in a Diabetic Patient: A Challenging Diagnosis. Clin Oncol. 2016; 1: 1108.
Abstract
Introduction: Tumor-induced hypoglycemia is a rare entity and it is mainly due to pancreatic
insulinomas. Non-islet cell tumor hypoglycemia is really exceptional and ectopic insulin secretion
has been previously suggested.
Case Presentation: A 79 year-old type 2 diabetic man, after over 30 years of poorly controlled
diabetes, observed an unexplained improving of glycemic control with recurrent hypoglycemia. He
progressively reduced insulin doses, till suspension, with persistent frequent hypoglycemia. A fasting
test documented symptomatic hypoglycemia with un appropriated elevated insulin and C-peptide.
CT scan and endoscopic ultrasound did not reveal any pancreatic lesion. A Ga68-Dotanoc showed
a focal pathological right pelvic uptake, corresponding to an oval enhancing lesion at the targeted
CT images. The patient was submitted to surgical excision of that mass, revealing an appendix
neuro endocrine well differentiated tumor with lymph nodes metastasis, showing partial insulin
immunohistochemistry staining. After surgery no other hypoglycemic events were documented; to
control diabetes insulin therapy needed to be reintroduced.
Conclusion: Extra-pancreatic insulin secreting tumors are very rare and their diagnosis in diabetic
patients can be challenging. This case addresses the diagnosis and treatment of this rare entity
reporting, to our knowledge, the first case of ectopic insulinoma, arising from appendix.
Keywords: Ectopic insulin secretion neuroendocrine tumor; Hypoglycemia; Diabetes
Introduction
The most frequent cause of hypoglycemia is a side effect of insulin or other anti diabetic drugs
overdose. In non diabetic subjects, hypoglycemia is usually due to endogenous insulin hyperproduction,
coming from nesidioblastosis or insulin secreting pancreatic tumors, arising from
islet β cells. On other rare occasions, hypoglycemia can occur as a consequence of auto-antibodies
towards insulin or its receptor that are mainly associated with para-neoplastic syndromes [1].
Tumor Induced Hypoglycemia (TIH) can rarely be developed by extra-pancreatic tumors and it
can be due to insulin ectopic secretion or to other neuroendocrine pathogenic mechanisms such as
IGF2, IGF1, somatostatin or GLP1 overproduction [2].
TIH, due to ectopic insulin secretion, usually results from duodenum or peri-pancreatic tumors.
The majority of these rare tumors come from ectopic accessory or aberrant pancreas [3]. Anyway,
anecdotal cases reported extra pancreatic insulin secreting tumors such as kidney neuroendocrine
tumor, bronchial carcinoid, cervix carcinomas, paraganglioma, schwannomma, and gastrointestinal
tumor [2].
Here in we report, to our knowledge, the first case of well documented appendix well
differentiated neuroendocrine insulin secreting tumor.
Case Presentation
A 79-year old man, diagnosed with diabetes in 1980 was treated with insulin for many years, with poor glycemic control. Suddenly, he developed recurrent
hypoglycemic episodes. Any relevant weight, diet or activities changes
were reported. As suggested by his diabetologist, the patient gradually
reduced, till suspension, the dose of insulin therapy, with persistent
recurrent hypoglycemias. After two weeks, since insulin interruption,
basal fast serum glycemia was 53 mg/dl, insulin 99 mUI/ml, C-peptide
10 ng/ml and Chromogranin A (without proton pump inhibitor or
other eventually interfering drugs) 435 ng/ml (Normal value 0-100
ng/ml). Thyroid function, serum cortisol and cathecolomines were
normal.
A 72h fasting test was started; it was stopped after 24h for a
symptomatic hypoglycemia (glycemia 38 mg/dl) associated with
unappropriated elevated insulin and C-peptide (Insulin 47.8 mUI/ml,
C-peptide 4.4 ng/ml) and with a pathological response to glucagon
(glycemia increasing from 38 to 90 mg/dl at 90 minutes after glucagon
infusion).
The patient was submitted to contrast enhanced abdominal CT
and to endoscopic ultrasound, without any documented pancreatic
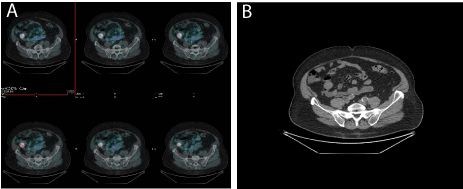
lesions. However, subsequent Gallium-68 Dotanoc Figure 1 showed a
focal pathological uptake in the right pelvic region that corresponded
to an oval pelvic mass, at the following targeted CT images.
He was submitted to abdominal laparoscopic surgery that
showed a cecal appendix mass that was removed together with local
lymph nodes. Pathology examination found an appendix of 5.5 cm
length with a diameter of 0.4-1.4 cm, surrounded by congested serosa.
Appendix lumen was occupied by a whitish necrotic lesion, infiltrating
the appendix wall but not the peri-appendix tissue. Microscopically it
was suggestive of a neuroendocrine appendix grading 2 tumor, with
necrosis, infiltrating the appendix wall till subserosa, with lymphatic
and vascular invasion and metastasis of one local lymph node
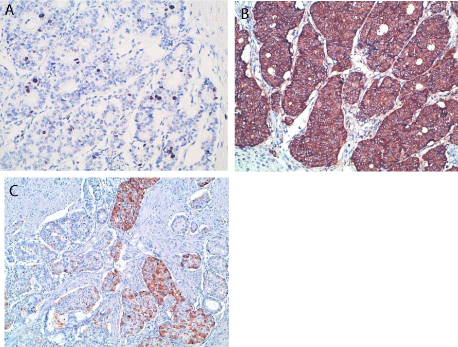
(pT2N1). Immunohistochemical staining revealed diffuse and intense
staining for chromogranin A, synaptophysin and partial positivity for
insulin. MIB1 labeling index was 10% and mitotic rate was 2 per 10
high power fields (Figure 2). The case was share in the Italian Rare
Cancer Network to obtain an expert pathological confirmation.
After surgery the patient did not presented any other
hypoglycemic episodes and, due to relapsed stable hyperglycemia,
he re introduced basal-bolus insulin treatment. Two months after
surgery serum chromogranin. A was normalized without any other
specific treatment. He will continue appropriate morphological and
biochemical follow up, to exclude any neoplastic recurrence.
Figure 1
Figure 1
Gallium-68 DOTANOC images showing focal pathological uptake in the right pelvic region, corresponding to an oval pelvic mass, at the targeted CT
images.
Figure 2
Discussion
Diabetic patients, especially if treated with insulin, are at high
risk of hypoglycemia. In diabetic patient, any hypoglycemic event
is generally ascribed to hypoglycemic therapy, thus the diagnosis of
insulinoma is more complex.
Incidence of diabetes in patients with insulinoma is lower
than in general population [4] and there are only sporadic case
reports of insulinoma in previously diabetic patients [5]. However,
when hypoglycemia persists after suspension of the hypoglycemic
treatment, an insulinoma must be suspected. Biochemical
diagnosis of hypoglycemia is established when a Whipple's triad
is documented (signs or symptoms of hypoglycemia, low plasma
glucose concentration (<40 mg/dl) and resolution of signs and
symptoms after glucose somministration). Hypoglycemia can
then be ascribed to insulinoma if a spontaneous hypoglycemia is
associated to concomitant insulin levels ≥6 μU/ml and C-peptide
levels ≥200 pmol/l (or proinsulin levels ≥5 pmol/l), with the absence
of sulfonylurea in the plasma or urine [6]. Alternatively, 72h fasting
test should be organized to document a fasting hypoglycemia with
concomitant in appropriated elevated insulin and C-peptide and/
or proinsulin levels [6]. At the end of 72h-fasting test, other useful
diagnostic tools, predictive of insulinoma are: beta-hydroxybutyrate
less than 2,7 mmol/l and a glucose response to 1mg glucagon iv
greater than 25 mg/dl [7].
In this case we documented a persistent recurrent hypoglycemia
in a patient with previously poorly controlled long term diabetes,
although the patient did not refer any life style or weight changes.
Elevated basal insulin, c-peptide and chromogranin a were suspected
for an insulinoma. This hypothesis was then confirmed after the 72h
fasting test during which a clear hypoglycemia was documented
and associated to insulin and C-peptide in the range suggestive of
insulinoma. Moreover, after glucagone test, glycemia increased of 52
mg/dl, corroborating the idea of insulinoma.
Insulinomas are almost universally located within the pancreas
(1/3 head–1/3 body–1/3 tail), although they are often so small that
their pre-surgical localization could become very difficult. As first
line radiological assessment, insulinomas are generally investigated
through abdominal ultrasound, CT or MRI [7]. Endoscopic
ultrasound can then help in localizing the pancreatic tumor, with a
positive imaging in 70–95% of cases.
Although only about 20-50% of insulinomas express somatostatin
receptors, an Octreoscan® or, more frequently, Gallium-DOTATOC
or DOTANOC can be useful to localize the tumor [8]. Moreover,
new nuclear imaging methods are recently been proposed, such as
scintigraphy using radiolabeled GLP-1 analogues, that seems to be
really promising in pre-surgical insulinoma localization. When all
the morphological investigations remain negative, selective arterial
calcium stimulation with hepatic venous sampling for insulin, can
help in localizing the tumor within the pancreatic head, corpus or
tail, before proceeding to an eventual surgical exploration, guided by
pancreatic manual palpation and intra-operative ultrasound.
In the reported case, abdominal CT and endoscopic ultrasound
did not find any pancreatic lesion. However, Gallium-DOTANOC
showed a pathological uptake in the right pelvic region, where the CT
images denoted an ovoid mass.
Hypoglycemia induced by non pancreatic tumor is a very
rare entity and its pathogenesis is not easily identified. Different
mechanisms have been previously proposed: (a) extrapancreatic
insulinoma; (b) ectopic insulin production; (c) increased consumption
of glucose by a tumor; (d) ectopic production of insulin-like peptide
(IGF2, IGF1) or GLP1.
Extrapancreatic insulinoma is an extremely infrequent condition,
reported in only 1-2% of all insulinomas and it has to be suspected
when a biochemically confirmed insulinoma cannot be localized
with radiological images. These very rare tumors have been mainly
reported to develop from ectopic pancreas, generally observed
in the duodenal wall or in the peri-pancreatic tissues [9]. Another
quite rare event is a non-islet cell tumor, causing an ectopic insulin
secretion such as it has been reported for some bronchial carcinoids,
kidney neuroendocrine tumor, paragangliomas, cervix carcinoma,
shwannoma and gastrointestinal stromal tumors.
In this case we are reporting a case of an appendix neuroendocrine,
grade 2 tumor, with an insulin positive immunohistochemistry.
This is to our knowledge, the first case of an appendix insulinsecreting
neuroendocrine tumor. We could only find a case report
of an appendix carcinoid with liver metastasis with reported signs of hyperinsulinemia and a weak and poorly expressed autoptical
immunohistochemistry reaction for insulin. However, in this
previously described case, a real hypoglycemia and a biochemical
diagnosis of insulinoma could not be done [10].
Surgery is the first line treatment for insulin secreting
neuroendocrine tumor, trying to perform a surgical resection, guided
from a radiological pre-operative localization or trying to find it out
with a surgical exploration.
This reported case underlines how an accurate pre-surgical
evaluation can identify even a really unusual or unique source
of ectopic insulin secretion, obtaining a complete control of
hypoglycemia and maybe a better prognosis.
Prognosis of these neuroendocrine tumors mainly depends upon
the grade and stage of the disease. Thanks to a quite prompt diagnosis,
after the beginning of symptoms, the grade 2 neuroendocrine tumor
was diagnosed before local and distant metastasis could become
clinically evident, permitting a likely radical surgery with a possible
complete and lastly neoplastic remission. Whenever a clinical
insulinoma is diagnosed, if localization with traditional and new
imaging cannot be obtained, a possible ectopic insulin secretion
should be supposed, trying to rule it out with the best now available
techniques such as Gallium-DOTATOC/DOTANOC or radiolabeled
GLP-1 analogues, in order to improve the patient prognosis.
References
- Cryer PR, Axelrod L, Grossman AB, Heller SR, Montori VM, Seaquist ER, et al. Evaluation and managenement of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2009; 94: 709-728.
- Iglesias P, Díez JJ. Management of endocrine disease: a clinical update on tumor-induced hypoglycemia. Eur J Endocrinol. 2014; 170: R147-R157.
- Hennings J, Garske U, Botling J, Hellman P. Malignant insulinoma in ectopic pancreatic tissue. Dig Surg. 2005; 22: 377-379.
- Levine RA, Sobel BE. Insulinoma, type 2 diabetes and plasminogen activator inhibitor type-I. Coron Artery Dis. 2001; 12: 333-336.
- Hameed MF, Hoyle GE, Muir Z. A mysterious case of normalising blood sugar: insulinoma in a long-standing diabetic patient. Age Ageing. 2006; 35: 317-318.
- Jensen RT, Cadiot G, Brandi ML, de Herder WW, Kaltsas G, Komminoth P, et al. ENETS Consensus Guidelines for the Management of Patients with Digestive Neuroendocrine Neoplasms: Functional Pancreatic Endocrine Tumor Syndromes. Neuroendocrinology. 2012; 95: 98-119.
- O'Brien T, O'Brien PC, Service FJ. Insulin surrogates in insulinoma. J Clin Endocrinol Metab. 1993; 77: 448-451.
- Sundin A, Garske U, Orlefors H. Nuclear imaging of neuroendocrine tumours. Best Pract Res Clin Endocrinol Metab. 2007; 21: 69-85.
- Hennings J, Garske U, Botling J, Hellman P. Malignant insulinoma in ectopic pancreatic tissue. Dig Surg. 2005; 22: 377-379.
- Urbanczyk K, Tomaszewska R. Cytological pattern of multihormonal carcinoid of the appendix with metastasis to the liver corresponding a clinical insulinoma. Pat Pol. 1994; 45: 225-229.