Mini Review
Postoperative Pancreatic Fistulas Complicated by Haemorrhage: Diagnosis and Treatment
Rösch Ch1, Gangl O1, Langer RM1, Gschwendtner M2 and Függer R1*
1Department of Surgery, Austria
2Institute for Radiology, Krankenhaus der Elisabethinen Linz, Austria
*Corresponding author: Reinhold Függer, Department of Surgery, Krankenhaus der Elisabethinen Linz, Fadingerstrasse 1, 4020 Linz, Austria
Published: 28 Sep, 2016
Cite this article as: Rösch Ch, Gangl O, Langer RM, Gschwendtner M, Függer R. Postoperative Pancreatic Fistulas Complicated by Haemorrhage: Diagnosis and Treatment. Clin Oncol. 2016; 1: 1105.
Abstract
Objective: We sought to review our experience with postoperative pancreatic fistulas complicated with bleeding.
Patients and Methods: Between January 2001 and September 2015 307 patients underwent
pancreatico-duodenoectomies or central pancreatectomies. All cares were reconstructed by
pancreaticojejunostomy.
Results: Overall 30 day mortality was 3.6% (11 of 307 patients). Seven patients (5 males and 2
females, 65.9 years (61-74)) had late post-pancreatectomy haemorrhage (PPH) with concomitant
pancreatic fistulas. Time from surgery to diagnosis was 16.3 days (range: 11-25.) CT, angiography
and gastroscopy were applied to diagnose the problem.
In all patients the eroded stump of the gastroduodenal artery was the underlying cause. Three patients
underwent surgery and four angiographic stenting for initial treatment. We lost 1 patient due to
repeated bleeding and multiple organ failure. The remaining 6 patients were hospitalized for 65
days (25-121) and recovered. During the study period our treatment strategy changed from primary
surgery to angiography with stenting. We also switched our technique of pancreaticojejunostomy
from one layer to duct-to-mucosa anastomosis and observed a reduction of incidence.
Conclusion: PPH is a life-threatening complication which can be treated preferably with
interventional techniques, surgery remains for salvage therapy.
Introduction
Continuous improvement of results in pancreatic surgery has been reported during the past two
decades. This was affected by improved surgical techniques, better management of complications and
a restriction of pancreatic surgery to centres with higher case numbers [1-3]. Although postoperative mortality dropped below 5%, morbidity remained high in a range of 40 - 50% [4,5]. Specific surgical complications as postoperative pancreatic fistula and post pancreatectomy haemorrhage (PPH)
account for the majority of morbidity. Especially the combination of pancreatic fistula and late post
pancreatectomy haemorrhage is potentially life-threatening. According to the International Study
Group of Pancreatic Surgery (ISGPS), such events are categorized as PPH Grade C [6]. Diagnostic
and therapeutic algorithms came in the focus, considering whether surgical, angiographic or
endoscopic procedures are optimal therapeutic strategies. Although reviews and case series indicate
a change from surgery to interventional radiology as the initial treatment, literature remains
controversial [7-10].
The aim of this analysis was to identify the incidence of pancreatic fistula complicated by
haemorrhage in our series, and to evaluate diagnostic and therapeutic strategies especially with
respect to possible changes during the study period.
Patients and Methods
A prospectively documented Pancreatic Surgery Data Base of our department was
retrospectively reviewed for patients undergoing pylorus preserving, classic Kausch-Whipple
pancreaticoduodenectomy or central pancreatic resection with postoperative pancreatic fistulas
combined with bleeding. Factors analyzed were underlying diagnosis indicating pancreatic surgery,
operative procedure, and technique of pancreatic anastomosis, time until diagnosis of pancreatic
fistula and bleeding, therapy and outcome. Regarding therapy, all percutaneous, angiographic,
endoscopic and surgical interventions were included for analysis. Additional interest was given to the incidence throughout the study period and possible changes in treatment modalities.
Between January 2001 and September 2015 a total of 307 patients
undergoing either pylorus preserving, classic Kausch-Whipple
pancreaticoduodenectomy or central pancreatectomies with
reconstruction by pancreaticojejunostomy was identified in our data
base and is subject of this analysis. 30–day mortality was 3.6% (11 of 307) overall.
Pancreatic fistulas complicated by bleeding were observed in 7
of 307 (2.3%) patients. There were five male and two female patients
with a mean age of 65.9 years (min 61-max 74 years).
Underlying pancreatic pathology were ductal pancreatic
adenocarcinoma (n=4), extrahepatic bile duct carcinoma (n=1),
IPMN (n=1) and microcystic adenoma (n=1). Six pylorus preserving
pancreaticoduodenectomy and one central pancreatectomy were
performed in these patients. Pancreaticojejunostomy was used
in all patients for reconstruction, either by classic single layer
anastomosis or by duct-to-mucosa anastomosis with selective use
of loose pancreatic duct drains since October 2007. The anastomotic
techniques applied are described in detail elsewhere [11].
Table 1
Table 1
Regarding treatment strategy, angiography with subsequent stenting replaced emergency relaparotomy as preferred initial procedure in 2006.
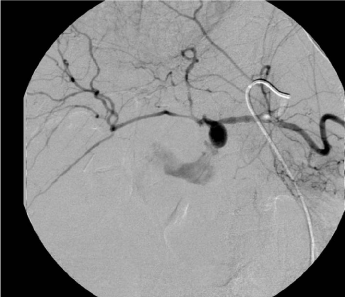
Figure 1
Figure 1
Localisation of bleeding from the stump of the gastroduodenal
artery in conventional angiography.
Results
Seven of 307 (2.3%) patients developed pancreatic fistula
complicated by bleeding. Time from pancreatic surgery to diagnosis
and therapy was 16.3 days in mean, with a range from 11 to 25
days. All patients presented with significant clinical deterioration,
especially signs of haemorrhage as haemodynamic instability,
haematemesis and melena. Following immediate haemodynamic
stabilisation, diagnosis was settled by computed tomography (6
of 7) and angiography (4 of 7) in most patients. Gastroscopy was
performed in only one patient in the early phase of this series. Six patients developed haemorrhage during their index hospitalisation.
One patient was discharged at postoperative day 10 following pylorus
preserving pancreaticoduodenectomy and readmitted because of a
late pancreatic fistula at day 15. He developed haemorrhage at day 25.
Three patients underwent relaparotomy as the first step in
complication management. In all of them, haemorrhage from an
eroded stump of the gastroduodenal artery was the underlying cause
of bleeding. Surgical haemostasis by suturing the stump and drainage
of the pancreatic fistula and fluid retentions were performed.
In the other four patients angiography was the first diagnostic
step. In all four, the stump of the gastroduodenal artery was identified
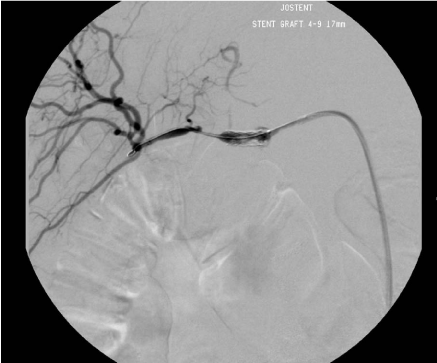
as source of haemorrhage and treated by overstenting in the same
procedure, which is shown in Figure 1 and 2. Additional percutaneous
CT guided drainage of a fluid retention was necessary in one patient
on the day of angiographic intervention.
There was no need for further angiographic, percutaneous or
surgical re-intervention in the four patients treated primarily by
angiographic stenting.
All three patients treated initially by relaparotomy were in need
for further interventions. While one patient underwent subsequently
percutaneous CT guided drainage of an abscess following
relaparotomy, two patients had multiple re-laparotomies due to
rebleeding and fluid retentions. Finally one patient died on day 39 from
the index operation (pylorus preserving pancreatoduodenectomy)
due to repeated bleeding and multiple organ failure. Thus mortality
of patients with pancreatic fistula complicated by bleeding was 14.3%
(one of 7).
Six patients survived with a hospitalisation of 65 days in mean
(range 25 to 121 days). Two patients with underlying diagnosis of
microcystic adenoma and pancreatic adenocarcinoma respectively,
are alive at late follow-up 4 and 11 years after pancreatic surgery. Three patients with pancreatic adenocarcinoma died due to tumor
recurrence and one patient (IPMN) was lost of follow-up.
Analysis of the distribution throughout the study period and
the technique of pancreatic anastomosis, revealed that 5 of 7 (71%)
of events emerged in the earlier period with single layer technique
and only two in the later period, when two layer duct-to-mucosa
anastomosis was used. In detail, fistulas complicated by bleeding
occured in 2004, 2006 (n=3), 2007, 2009 and 2011. Regarding
treatment strategy, angiography with subsequent stenting replaced
emergency relaparotomy as preferred initial procedure in 2006. Since
then, only the readmitted patient underwent primary relaparotomy
(Table 1).
Figure 2
Discussion
In the literature an incidence of late PPH between 3-6% is
reported. [9,12,13]. In our series 2.3% of patients undergoing
pancreaticoduodenectomy or central resection developed late lifethreatening
bleeding. Most bleedings are seen in the second week from
index operation, well corresponding to our findings of 16 days. Late
occurrence is an indicator of the predominant role of postoperative
pancreatic fistula and infection. Pancreatic fistula has been found
to be the most striking risk factor for late PPH [7-9,13,14]. Roulin
et al. [12] report in their series, that 62% of patients with delayed
massive bleeding had pancreatic fistulas. In our patients, late PPH
of Grade C was always accompanied by a pancreatic fistula. In our
opinion, nearly all patients with PPH Grade C have a concomitant
pancreatic fistula as a trigger for haemorrhage [15]. Difficulties in
the verification of pancreatic fistulas in the later course, especially in
the absence of drains or larger fluid retentions and varying patient
selection may cause underestimation of the incidence of pancreatic
fistula in reported series.
Mortality is high with a range from 13-30% [7-9,13,14,16,17] and
was 14.3% in our series. Aside successful treatment of bleeding, it is
crucial to drain the pancreatic fistula and evacuate abscesses and fluid
retentions. Failure of source control will impair prognosis [13].
Surgery, angiography and endoscopy are reported as possible
treatment modalities. Recently published case series cover study
periods of 10 to 15 years. In most of them relaparotomy was the initial
therapy in the early phase, currently being replaced by angiographic
intervention. In our patients, relaparotomy was the preferred
therapy in the early years. Due to unsatisfying results we changed
to angiography as primary diagnostic and therapeutic tool in 2006.
Rather the same clinical experience may have caused this shift from
surgery to interventional radiology in most centers, due to a lack of
high level studies proving the optimal strategy. Literature is restricted
to case series and reviews. Khalsa reports 100% mortality following
relaparotomy compared with 25% for the combination of surgery
and angiography and 0% for angiography alone [8]. A decrease in
mortality by the shift from surgery to angiography was also observed
by Ansari and Roulin [7,12]. Regarding control of bleeding, 75%
success rates for surgery and 100% for angiografic embolization
were indicated [7]. In contrast, initial surgical revision remains high
with 28% compared to 35% angiographic, 22% endoscopic and 28%
conservative therapy in other series [10]. Missing comparability and
individual patient selection may explain these differences.
For angiographic control of haemorrhage, either embolization or
covered stents are used. Recently, Hassold reported decreased 30-day
mortality (19% vs. 30%) for stenting. Stenting was also recommended because of less ischemic complications compared with embolization [16].
Sentinel bleeding as a warning before life–threatening massive
haemorrhage is described in 45% - 63% [7,12]. In combination with
an existing pancreatic fistula, each sentinel bleeding should cause
prompt and consequent diagnostic work-up by CT and angiography.
We advice angiographic stenting in these patients, if the source of
sentinel bleeding can be localized.
Most often the stump of the gastroduodenal artery is the source
of haemorrhage. In our experience, keeping the stump long during
resection is of help for stent placement and saves the integrity of the
hepatic artery.
Endoscopy is of minor priority in patients with
pancreaticojejunostomy, but of crucial importance controlling bleeding
from the pancreatic remnant following pancreaticogastrostomy. In
our opinion this scenario represents another pathology with demand
of different strategies.
Finally we found, that more events in our series were observed in
the period of single layer pancreaticojejunostomy. While five patients
with single layer anastomosis suffered from pancreatic fistula and
late PPH Grade 3, only 2 patients were diagnosed since we changed
to duct-to-mucosa pancreaticojejunostomy. The debate whether an
optimal pancreatic anastomosis exists is long and unsolved. Regarding
pancreatic fistulas combined with haemorrhage, no difference was
found [18]. We also cannot deviate an advantage for the duct-tomucosa
anastomosis regarding the incidence of pancreatic fistula
and bleeding from our data. However, due to an unfavorable series
of events in 2006, we decided to change our anastomotic technique.
In a case match study comparing our anastomotic techniques, we
found a reduction of unplanned interventions with duct-to-mucosa
technique [11]. This may be considered an argument for duct-tomucosa
anastomosis.
In conclusion, pancreatic fistula combined with haemorrhage is
a life threatening complication with need for consequent and prompt
diagnostic and therapeutic work-up. Following initial haemodynamic
stabilisation, angiographic overstenting and percutaneous drainage
of fluid retentions is our preferred treatment mode. Surgery is the
salvage option in case of failure of interventional techniques.
References
- Nathan H, Cameron JL, Choti MA, Schulick RD, Pawlik TM. The Volume-Outcomes Effect in Hepato-Pancreato-Biliary Surgery: Hospital Versus Surgeon Contributions and Specificity of the Relationship. J Am Coll of Surg. 2009; 208: 528-538.
- McPhee JT, Hill JS, Whalen GF, Zayaruzny M, Litwin DE, Sullivan ME, et al. Perioperative Mortality for Pancreatectomy: A National Perspective. Ann Surg. 2007; 246: 246-253.
- Lemmens VE, Bosscha K, van der Schelling G, Brenninkmeijer S, Coebergh JW, de Hingh IH. Improving outcome for patients with pancreatic cancer through centralization. British Journal of Surgery. 2011; 98: 1455-1462.
- Witzigmann H, Diener MK, Kienkötter S, Rossion I, Bruckner T, Bärbel Werner, et al. No Need for Routine Drainage After Pancreatic Head Resection: The Dual-Center, Randomized, Controlled PANDRA Trial (ISRCTN04937707). Ann Surg. 2016; 264: 528-537.
- Gangl O, Sahora K, Kornprat P, Margreiter C, Primavesi F, Bareck E, et al. Preparing for Prospective Clinical Trials: A National Initiative of an Excellence Registry for Consecutive Pancreatic Cancer Resections. World J Surg. 2014; 38: 456-462.
- Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, et al. Postpancreatectomy hemorrhage (PPH)–An International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007; 142: 20-25.
- Ansari D, Tingstedt B, Lindell G, Keussen I, Ansari D, Andersson R. Hemorrhage after Major Pancreatic Resection: Incidence, Risk Factors, Management, and Outcome. Scand J Surg. 2016.
- Riediger H, Krüger K, Makowiec F, Adam U, Krueger CM. [Symptoms, Diagnostics, Treatment and Classification of 22 Patients with Postpancreatectomy Haemorrhage (PPH) in a Series of 400 Consecutive Pancreatic Head Resections and Pancreatectomies]. Zentralbl Chir. 2016.
- Khalsa BS, Imagawa DK, Chen JI, Dermirjian AN, Yim DB, Findeiss LK. Evolution in the Treatment of Delayed Postpancreatectomy Hemorrhage: Surgery to Interventional Radiology. Pancreas. 2015; 44: 953-958.
- Feng J, Chen YL, Dong JH, Chen MY, Cai SW, Huang ZQ. Postpancreaticoduodenectomy hemorrhage: risk factors, managements and outcomes. Hepatobiliary Pancreat Dis Int. 2014; 13: 513-522.
- Gangl O, Fröschl U, Langer RM, Függer R. Single-layer versus duct-to-mucosa pancreaticojejunostomy in pyloruspreserving pancreatoduodenectomy for ductal adenocarcinoma—an analysis of a single surgeon’s series. European Surgery. 2016; 48: 34-38.
- Roulin D, Cerantola Y, Demartines N, Schäfer M. Systematic review of delayed postoperative hemorrhage after pancreatic resection. J Gastrointest Surg. 2011; 15: 1055-1062.
- Asari S, Matsumoto I, Toyama H, Yamaguchi M, Okada T, Shinzeki M, et al. Recommendation of treatment strategy for postpancreatectomy hemorrhage: Lessons from a single-center experience in 35 patients. Pancreatology. 2016; 16: 454-463.
- Darnis B, Lebeau R, Chopin-Laly X, Adham M. Postpancreatectomy hemorrhage (PPH): predictors and management from a prospective database. Langenbecks Arch Surg. 2013; 398: 441-448.
- Gangl O, Fröschl U, Hofer W, Huber J, Sautner T, Függer R. Unplanned reoperation and reintervention after pancreatic resections: an analysis of risk factors. World J Surg. 2011; 35: 2306-2314.
- Asai K, Zaydfudim V, Truty M, Reid-Lombardo KM, Kendrick M, Que F, et al. Management of a delayed post-pancreatoduodenectomy haemorrhage using endovascular techniques. HPB (Oxford). 2015; 17: 902-908.
- Hassold N, Wolfschmidt F, Dierks A, Klein I, Bley T, Kickuth R. Effectiveness and outcome of endovascular therapy for late-onset postpancreatectomy hemorrhage using covered stents and embolization. J Vasc Surg. 2016.
- Eckardt AJ, Klein F, Adler A, Veltzke-Schlieker W, Warnick P, Bahra M, et al. Management and outcomes of haemorrhage after pancreatogastrostomy versus pancreatojejunostomy. Br J Surg. 2011; 98: 1599-607.