Research Article
Outcomes of Surgery in Elderly Patients with a Retroperitoneal Soft Tissue Sarcoma
Macovei R1, Sourouille I1, Levy A2, Mir O3, Ceribelli C1, Terrier P4, LePéchoux C2, LeCesne A2 and Honoré C1*
1Department of Surgical Oncology, Gustave Roussy Cancer Campus, France
2Department of Radiotherapy, Gustave Roussy Cancer Campus, France
3Department of Pathology, Gustave Roussy Cancer Campus, France
4Department of Medical Oncology, Gustave Roussy Cancer Campus, France
*Corresponding author: Honoré C, Department of Surgical Oncology, Gustave Roussy Cancer, Campus114, rue Edouard vaillant, 94805, Villejuif Cedex, France
Published: 28 Sep, 2016
Cite this article as: Macovei R, Sourouille I, Levy A, Mir O, Ceribelli C, Terrier P, et al. Outcomes of Surgery in Elderly Patients with a Retroperitoneal Soft Tissue Sarcoma. Clin Oncol. 2016; 1: 1102.
Abstract
Aim of this Study: To evaluate short and long term results after curative surgery for a retroperitoneal
sarcoma (RPS) in elderly patients.
Methods: We retrospectively analyzed data of all patients operated in our single tertiary care center
for a non-metastatic RPS and identified patients older than 70.
Results: Among 304 patients with a RPS treated between 1994 and 2015, 62 (20%) were older than
70 (median age 75 years, range: 70-85). The median tumor size was 26 cm (range: 11-46). 46 patients
(74%) had mass-related symptoms at the time of diagnosis. The most frequent histological subtype
was liposarcoma (76%). 22 patients (35%) had a perioperative radiotherapy and/or chemotherapy.
58 patients (94%) had macroscopically complete resection. The postoperative mortality was 6% and
severe morbidity (including deceased patients) was 39%. A reoperation was required for 11 patients
(18%). After a median follow-up of 20 months (range: 0-120), the 5-year Overall Survival (OS) rate
was 90% (IC95%: 79%-100%) and the median OS was not reached. Cancer specific death rate was
86%. No prognostic factor for disease specific survival was detected. The 5-years disease free survival
DFS rate was 52% (IC 95%: 33%-84%) and the 5-years loco-regional recurrence-free survival (LRRFS)
rate was 52% (IC 95%: 33%-84%). Median DFS was 94 months (range: 35-139). Reoperation
after inappropriate surgery and postoperative morbidity were independent predictive factors of
loco-regional relapse. No predictive factors of distant metastasis were found.
Conclusions: Curative surgery is feasible in selected elderly patients with an acceptable morbidity
and with potential action on symptoms. It enables a prolonged survival. Future studies should focus
on selection process.
Keywords: Soft tissue sarcoma; Surgery; Elderly; Cancer; Retroperitoneal sarcoma
Introduction
Soft Tissue Sarcomas (STS) account for 2% of all adult cancers [1]. With an estimated incidence of 59 per million and per year, approximately 30,000 new cases are diagnosed yearly in Europe, 11,900 in the United States and 2000 in Japan [1-3]. Fifteen percent of all STS are located in the retro-peritoneal space [1-2]. Surgery is the reference treatment for non-metastatic retro-peritoneal soft tissue sarcomas (RPS). The extent of surgery is still debated. An international multi-centric series on 1007 patients have recently shown better results, attributed partially to a more aggressive surgical approach consisting in a systematic in-bloc resection with adjacent viscera even when not overtly involved [4]. With this approach, 5 years overall survival (OS) was 67%, and the median survival 116 months [4]. Nevertheless, few data exist on the potentially increased morbidity associated with multivisceral resection, especially in elderly patients. The aim of this study was to evaluate short and long term results after excision of RPS in patients aged over 70 years, to determine if surgery should be proposed to these patients.
Methods
Patient’s selection
We retrospectively analyzed all patients operated for a primary RPS in our single tertiary care center between November 1994 and October 2015 to identify patients
older than 70 years. Inclusion criteria were (i) data available on initial treatment and follow-up and (ii) no concomitant uncontrolled other
cancer. We excluded patients with non-sarcoma pathology, as well as patients with solitary fibrous tumors, or with uncertain malignancy.
Patient’s files were retrospectively analyzed.
Variables analyzed
The analyzed variables were preoperative data (gender, age,
tumor location, size, symptoms), peroperative data (date of surgery,
resection performed, mortality and postoperative morbidity,
histology, microscopic margins, perioperative radiotherapy and/
or chemotherapy) and long-term data (overall survival, disease-free
survival, type of recurrence, death).
Preoperative work-up
All patients with RPS scheduled for surgery had a clinical
evaluation examination, a preoperative thoraco-abdomino-pelvic
CT-scanner and an abdomino-pelvic MRI when required on the
surgeon’s advice. Patients with metastatic disease on imaging or
with poor general status (i.e. ECOG 3-4) were considered unfit for
surgery. All tumors had a preoperative needle core biopsy with a
14- or 16-gauge using an imaging-guided coaxial technique. The
loco-regional contra-indications for surgery were based on technical
criteria reported by the EORTC-STBSG in 2012 [5].
Surgical technique
Surgery was performed according to the 2012 consensus
statements from the EORTC-STBSG European and North American
expert sarcoma surgeons [5]. The quality of the tumor resection
was defined according to the UICC criteria. Tumor rupture during
surgery and incomplete resection were recorded.
Postoperative morbidity
Surgical complications during the hospitalization were
retrospectively recorded and graded according to the Dindo/Clavien
classification [6]. A post-operative complication was considered
significant when the grade was greater than 2.
Pathological staging
All surgical specimens were analyzed and retrospectively
converted according to the 2012 WHO classification, with a further
molecular analysis whenever necessary. The pathology analysis
included the tumor grading using the FNCLCC classification and the
UICC TNM staging system [7-9].
Long term follow-up
Patients were followed with clinical examination and abdominopelvic
CT-scanner twice a year for 5 years and yearly afterwards.
Recurrences were diagnosed either on a clinical or radiological basis,
without required histological proofs, and systematically confirmed
with a multidisciplinary team decision.
Statistics
We calculated Overall survival (OS), time to local recurrence, and
time to metastasis using the Kaplan-Meier method and computed
confidence intervals with Rothman’s method. OS was computed
from the date of primary tumor resection to the date of death or the
last follow-up. Patients with postoperative death were excluded from
survival analysis. Time to local and time to distant recurrence were
computed from the date of primary tumor resection to the date of local
or distant recurrence. Local recurrences were ignored for the analysis
of distant recurrences and vice versa. For both abdominal and distant
recurrences analyses, death was considered as a censoring event.
Univariate and multivariate prognostic analyses were performed for
OS, abdominal and distant recurrences using the log-rank test and Cox proportional hazards models. The variables found statistically
significant variables in any of the univariate analysis were retained in
the multivariate analyses. Multivariate analyses were stratified on sex,
age (< >50 years), and tumor size (< >20cm). Variables were selected
with a backward selection algorithm; relative risks are given with
their 95% confidence intervals. All tests were two-sided and the 5%
significance level was used.
Table 1
Table 2
Results
Between 1994 and 2015, among the 304 patients operated in our
center for a primary RPS, 62 patients (20%) were older than 70.
Demographics and perioperative treatment
Patient characteristics of all 304 patients are given in Table 1.
Considering only the subset of patients older than 70, the median
age was 75 (range: 70-85). The median tumor size was 26 cm [range
11-46]. Forty-six patients (74%) had mass-related symptoms at the
time of diagnosis (pain, increased abdominal perimeter, dysuria,
constipation, gastro-esophageal reflux). Five patients (8%) had a
RPS extended in the lower limb. The most frequently encountered
histological subtype was liposarcoma (76%). Chemotherapy was
given preoperatively to 6 patients (10%) and postoperatively to 1 (2%).
Fifteen patients (24%) had radiotherapy, 7 (11%) preoperatively and
8 (13%) postoperatively. 54 patients (87%) had a first tumor resection
and 8 patients (13%) had a second surgery after inadequate resection
outside our tertiary care center. Compared to the younger population,
less patients older than 70 had preoperative chemotherapy (p=0.009)
or postoperative radiotherapy (p=0.024) (Table 1).
Type of surgery
At the time of surgery, 2 patients (3%) were deemed unresectable
and underwent exploratory laparotomy (one patient because of the intraoperative findings of a vascular invasion of the iliac vessels
and the other because of the intraoperative findings of an extensive
synchronous peritoneal sarcomatosis). Sixty patients underwent
resection and 58 (94%) had a macroscopically complete tumor
removal. The reasons for incomplete resection were an intra-thoracic
extension of the disease with a close contact to the aorta and a massive
unexpected peroperative bleeding from a lumbar vein wound that
required a shortened laparotomy. No tumor rupture was recorded
in patient after complete resection. The surgery was performed
without any organ resection in 6% of patients, with the resection
of one organ in 8% and with the resection of multiple organs in
84%. Colon-rectum and kidney were the most frequently resected
organs, in 84% of patients. Concerning resection with the potential
for high postoperative morbidity, 4 patients (6%) had a splenopancreatectomy,
3 patients (5%) had great nerve resection, and 2
patients (3%) had great vessels resection (iliac vein in both cases). No
patient required a definitive stoma, either digestive or urinary. The
mean peroperative blood losses were 954ml (range: 15-5000) and 21
patients (34%) required a perioperative transfusion.
Postoperative morbidity and mortality
The in-hospital mortality was 6% (n=4). The causes of death
were postoperative peritonitis after digestive anastomotic fistulas
(n=2), massive air embolism after a peroperative inferior cava vein
wound (n=1), and coma without identified etiology (n=1). Severe
postoperative morbidity (including deceased patients) was 39%
(n=24) and complications are listed in Table 2. An emergent surgery
was required for 11 patients (18%). No patient had postoperative
renal failure requiring hemodialysis. Compared to the younger
population, higher rates of postoperative morbidity (p=0.020) and
mortality (p=0.033) were recorded in patients older than 70.
Survival
After a median follow up of 20 months (range: 0-120), the 1-year,
3-year and 5-year OS rate were respectively 100% (IC95%: 100%-
100%), 90% (IC95%: 79%-100%) and 90% (IC95%: 79%-100%). The
median OS was not reached. At the last update, 8 patients had died.
Cancer specific death rate was 86% (one patient died of unknown
cause). No prognostic factors for disease specific survival were
detected in the univariate and multivariate analysis.
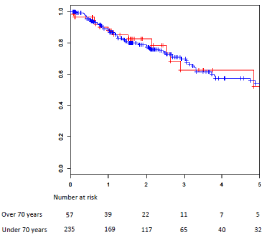
Compared to younger patients, identical rates of 5-years OS (p=0.23) were recorded in patients older than 70. The results are
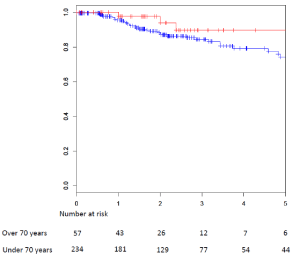
illustrated in (Figure 1 and 2).
Recurrence
The 1-year, 3-year and 5-year DFS rate were respectively 88%
(IC95%: 79%-98%), 63% (IC95%: 46%-85%) and 52% (IC 95%:
33%-84%). Median DFS was 94 months (range: 35-139). Thirteen
patients had a loco-regional relapse among which 6 had an associated
peritoneal sarcomatosis. The 5-years loco-regional recurrence-free
survival (LR-RFS) rate was 52% (IC 95%: 33%-84%). The relapse
was treated with chemotherapy in 7 patients, with radiotherapy in
3 patients and with best supporting care in 3 patients. Three patients
developed distant metastasis. Distant metastasis were located in the
lung (n=2) and in the liver (n=1). The rate of distant metastases
occurrence at 5-years was 5% (IC95%: 0%-12%). In univariate analysis,
factors significantly associated with loco-regional relapse in patients
older than 70 were reoperation after inappropriate initial surgery
(p=0.036), positive microscopic margins (p=0.023) and postoperative
morbidity (p=0.045). In multivariate analysis, reoperation after
inappropriate initial surgery (p=0.002) and postoperative morbidity
(p=0.020) remained independent loco-regional relapse predictive
factors. No predictive factor of distant metastasis or sarcomatosis was
found. Compared to younger patients, identical rates of 5-years locoregional
relapse free survival (p=0.18) were recorded in patients older
than 70. The results are illustrated in (Table 1), and (Figure 1 and 2).
Octogenarian patients
Eleven patients older than 80 were operated for a RPS in our
series. Median age was 82 (range: 80-85). All patients had a first
tumor resection. None of them received a preoperative treatment.
The postoperative mortality was 18% (n=2) and the morbidity
was 45% (n=5). Five patients (45%) required an emergent surgery
(2 postoperative peritonitis for anastomotic fistulas, 2 bowel
obstructions, 1 infected abdominal wall prosthetic mesh). One
patient had postoperative radiotherapy, no patient had postoperative
chemotherapy. One patient developed distant metastasis after 94
months (lung). No patient older than 80 died after hospital discharge
after a median follow-up of 24months [1-117].
Figure 1
Figure 2
Discussion
We report a series of 62 selected elderly patients operated fora
RPS, with a median tumor size of 26cm, among whom loco-regional recurrence rates and OS rates were comparable to those of a younger
population. The postoperative in-hospital morbidity and mortality in
this elderly population fit for surgery was respectively 39% and 6%.
Population aging and geriatric oncology
The proportion of the elderly population is rapidly increasing
in developed countries. Today in France, the life expectancy is 79
years for French men and 85 years for French women [10]. In 2040,
people older than 80 could exceed 9% of the population [11]. With
this aging of the population, we face new challenges. Nearly a third
of all cancers occur in people older than 75 and we can expect an
increased incidence of cancer in the next few years [12]. Even if
there is no evidence based cut-off age to define an "elderly" situation,
70 to 75 years-old is often considered an arbitrary threshold in
medical oncology. We encounter in this population specific elderly
frailty, both medical and social, we need to cope with when treating
these patients [12]. STS are not different from other cancer as their
incidence is also increasing with age, even if the histological subtypes
vary [13]. RPS represents a tremendous challenge because major
abdominal surgery with multivisceral resection is the cornerstone of
the curative treatment.
These consequences of this aggressive must be balanced when
treating older patients with associated comorbidities and with a
potentially increased postoperative morbidity and mortality [14].
Elderly patient’s selection for surgery
Aging may be defined as a progressive decline in the functional
reserve of multiple organ systems. This process is highly individualized,
and poorly reflected in chronological age. The treatment of cancer
should be based on the assessment of the physiological age, the
patient's life expectancy and tolerance to the treatment. Physiological
rather than chronological age should determine the management of
cancer in each individual [15]. Of the various instruments proposed
for the assessment of physiological age, a Comprehensive Geriatric
Assessment (CGA) is the most reliable, as both cancer-independent
mortality risk and functional reserve may be estimated based on the
CGA (inability to perform the Activities of Daily Living (ADL) and
the Instrumental Activities of Daily Living (IADLs), the presence of
multiple morbidities, the cognitive status, the presence of geriatric
syndromes, the nutritional status and the social support of the
patient). In particular, the benefits of cancer treatment diminish with
increased risk of non-cancer related mortality and of therapeutic
complications. Comorbidity and functional status influence both [16-
19]. With respect to the functional status, the ability to perform the
basic Activities of Daily Living (ADL) and the Instrumental Activities
of Daily Living (IADL) should be assessed in addition to traditional
oncological measures of function, such as the Karnofsky scale and
Eastern Cooperative Oncology Group (ECOG) performance status
[20-21]. At present, there is no universal screening test that adequately
identifies frailty in at risk older patients and most score were not
available at the beginning of this study [22]. Selection process was
prospectively based on ECOG scale and patient with a score less
than 2 were deemed fit for surgery. In that selected population, we
demonstrated that long survival could be achieved with an acceptable
postoperative mortality. In the near future, the use of the new test
could help to refine the patient selection for treatment.
Type of surgery and morbidity/mortality balance
Surgery enables the best results in non-metastatic RPS, with a 5
years OS of 67%, and a median survival of 116 months [4].
Because we found no difference between 5-years OS rates between older and younger patients in our series,
we could expect the same results as in the literature when performing surgery on fit elderly patients.
We found nevertheless higher rates of postoperative morbidity and
mortality in patients older than 70 and that postoperative morbidity
was an independent loco-regional relapse predictive factor. Decreased
performance status and significant comorbidities could therefore
determine the type of surgery, with a less extensive resection (i.e.
colonic/digestive tract sparing) in frailer patient [23]. On the
other hand, fit patient with a good life expectancy should undergo
systematic in-bloc resection with adjacent viscera even when not
overtly involved. In our series, complete surgery was possible with
the resection of at least 2 organs in the most of the cases (84%). This
aggressive surgical attitude could explain our good long term results,
and even if mortality is increased, it remains acceptable compared to
other cancer surgery frequently performed in elderly patients [24].
The studies who identified age as a significant independent prognostic
factor could have been suffering from the bias of under-treatment in
this elderly population [23]. One often neglected but important factor
to also integrate when deciding the best treatment is the action of
surgery on the symptom in these larges tumors.
Alternative treatments to surgery
Studies report the underuse of radiotherapy and chemotherapy
in elderly patients [24-26]. In many prospective clinical trials, these
patients are not included because of restrictive selection criteria.
Recent studies nevertheless demonstrated that chemotherapy and
radiotherapy could be well tolerated by elderly patients and was
beneficial [26,27]. The same remark can be made for surgery, as
cancer-directed surgery was demonstrated underused in elderly
patients in a recent nation-wide american database, even in localized
disease [26]. This study reported a significantly decreased odd of
receiving surgery beginning at 60 years for lung cancer, at 70 years for
liver cancer, and at 80 years for pancreatic cancer [26]. In our series,
even if no difference concerning the survival was significant between
the younger and the elderly population, we found less preoperative
chemotherapy and less postoperative radiotherapy in the group older
than 70. The natural life-expectancy at the actual age is a key factor
to take in account when deciding a treatment. People often think of
elderly patients as nearly in the grave when they are in fact survivors.
In France, natural life expectancy at 75 is 14 years for a man and 18
years for a woman [28]. In our series, median survival after surgery
was not reached after 20 months of median follow-up and only one out
of 9 deceased patients died of non-cancerous cause. As an alternative
to surgery, the reference chemotherapy in STS is since 30 years based
on doxorubicin with/without ifosfamide, with a median OS in a
metastatic setting of 13 months when using doxorubicin alone and
14months when using a combination of doxorubicin and ifosfamide
[29]. Unfortunately, these toxic regimens can barely be used in elderly
patients. Metronomic oral cyclophosphamide plus prednisolone is a
seducing alternative in elderly patients with inoperable or metastatic
STS [25]. With a good toxicity profile, it enabled a median OS of 14
months. There are few reports on irradiation results for inoperable
RPS patients. In a phase II assessing carbon ion therapy in a limited
dataset of unresected patients, outcomes were favorable (3 years
actuarial overall survival and local control rates of 73% and 63%,
respectively) and no severe acute and late reactions were reported [30].
In the absence of such technique Intensity Modulated Radiotherapy
(IMRT) should be the preferred treatment modality as it could help
reducing the high-dose irradiated volume within the intestinal cavity, including the contralateral kidney [31]. Anyway, every decision of
treatment in geriatric oncology should respect the four fundamental
ethical principles (benevolence, non-maleficence, equity, autonomy)
but the maintenance of an ethical reflection should not become a
pretext for a systematic under-treatment [29].
Strengths and weaknesses
This study suffers several biases. Besides its retrospective design,
we only studied patients deemed fit for surgery and could not
evaluate unfit/inoperable patients. The performance status is missing
as well as the ASA score. Nevertheless, we were able to identify a large
population of elderly patients operated for a RPS in a high volume
specialized center and were able to demonstrate the feasibility of
major abdominal surgery and long term survival.
Conclusion
Complete resection with adequate surgical margins for RPS is feasible in selected elderly patients with an acceptable postoperative morbidity and mortality. It potentially enables longer survival than chemotherapy alone with a direct action on symptoms. Future studies should focus on selection process.
References
- La situation du cancer en France en collection rapport et synthèses. Ouvrage collectif éditépar l’INCa, Boulogne-Bilancourt. 2010; 193-195.
- Ducimetière F, Lurkin A, Ranchère-Vince D, Decouvelaere AV, Péoc'h M, Istier L, Chalabreysse P, et al. Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS One. 2011; 6: e20294.
- ESMO/European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014; 25: iii102-112.
- Gronchi A, Strauss DC, Miceli R, Bonvalot S, Swallow CJ, Hohenberger P, et al. Variability in Patterns of Recurrence After Resection of Primary Retroperitoneal Sarcoma (RPS): A Report on 1007 Patients From the Multi-institutional Collaborative RPS Working Group. Ann Surg. 2016; 263: 1002-1009.
- Bonvalot S, Raut CP, Pollock RE, Rutkowski P, Strauss DC, Hayes AJ, et al. Technical considerationsin surgery for retroperitoneal sarcomas: position paper from E-surge, a master class in sarcoma surgery, and EORTCSTBSG. Ann SurgOncol. 2012; 19: 2981-2991.
- Dindo D, Demartines N, Clavien P. Classification of surgical complications: a new proposal with evaluation in a cohort pf 6336 patients and results of a surgery. Ann Surg. 2004; 240: 205-213.
- Gronchi A, Miceli R, Shurell E, Eilber FC, Eilber FR, Anaya DA, et al. Outcome prediction in primary resected retroperitoneal soft tissue sarcoma: histology-specific overall survival and disease-free survival nomograms built on major sarcoma center data sets. J ClinOncol. 2013; 31: 1649-1655.
- Russell WO, Cohen J, Enzinger F, Hajdu SI, Heise H, Martin RG, et al. A clinical and pathological staging system for soft tissue sarcomas. Cancer. 1977; 40: 1562-1570.
- Trojani M, Contesso G, Coindre JM, Rouesse J, Bui NB, de Mascarel A, et al. Soft tissue sarcomas of adults; study of pathological prognosis variables and definition of a histopathological grading system. Int J Cancer. 1984; 33: 37-42.
- [Évolution de l'espérance de vie à divers âges jusqu'en 2015].
- Leon O. Projections départementales et régionales de population à l'horizon 2040.
- Mohile SG, Hurria A, Cohen HJ, Rowland JH, Leach CR, Arora NK, et al. Improving the quality of survivorship for older adults with cancer. Cancer. 2016; 122: 2459-2568.
- Honoré C, Méeus P, Stoeckle E, Bonvalot S. Soft tissue sarcoma in France in 2015: Epidemiology, classification and organization of clinical care. J Visc Surg. 2015; 152: 223-230.
- Charpin J. Perspectives démographiques et financières de la dépendance. 2011.
- Brighi N, Balducci L, Biasco G. Cancer in the elderly: is it time for palliative care in geriatric oncology? J Geriatr Oncol. 2014; 5: 197-203.
- Charlson ME, Pompei P, Ales KL, Mackenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987; 40: 373–383.
- Yourman LC, Lee SJ, Shomberg MA, Widera EW, Smith AK. Prognostic indices for older adults. JAMA. 2012; 307: 182–192.
- Hurria A. Embracing the complexity of comorbidity. J ClinOncol. 2011; 32: 4217–4218.
- Extermann M, Boler I, Reich RR, Lyman GH, Brown RH, DeFelice J, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2012; 13: 3377–3386.
- Extermann M, Overcash J, Lyman GH, Parr J, Balducci L. Comorbidity and functional status are independent in older cancer patients. J ClinOncol. 1998; 16: 1582–1587.
- Repetto L, Fratino L, Audisio RA, Venturino A, Gianni W, Vercelli M, et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: an Italian Group for Geriatric Oncology Study. J ClinOncol. 2002; 20: 494–502.
- Pamoukdjian F, Paillaud E, Zelek L, Laurent M, Lévy V, Landre T, et al. Measurement of gait speed in older adults to identify complications associated with frailty: A systematic review. J Geriatric Oncol. 2015; 6: 484-496.
- Lahat G, Dhuka AR, Lahat S, Lazar AJ, Lewis VO, Lin PP, et al. Complete soft tissue sarcoma resection is a viable treatment option for select elderly patients. Ann Surg Oncol. 2009; 9: 2579-2586.
- Landi F, Vallribera F, Rivera JP, Bertoli P. Morbidity after laparoscopic and open rectal cancer surgery: a comparative analysis of morbidity in octogenarians and younger patients. Colorectal Dis. 2016; 5: 459-67.
- Mir O, Domont J, Cioffi A, Bonvalot S, Boulet B, Le Pechoux C, et al. Feasibility of metronomic oral cyclophosphamide plus prednisolone in elderly patients with inoperable or metastatic soft tissue sarcoma. Eur J Cancer. 2011; 4: 515-519.
- O'Connell JB, Maggard MA, Ko CY. Cancer-directed surgery for localized disease: decreased use in the elderly. Ann SurgOncol. 2004; 11: 962-969.
- Desbat NH, Levy A, Auberdiac P, Moncharmont C, Oriol M, Malkoun N, et al. Curative-intended treatment of squamous cell anal carcinoma in elderly adults. J Am Geriatr Soc. 2012; 60: 1993-1994.
- Esperance-de-vie-statistique-calcul-viager.
- Judson I, Verweij J, Gelderblom H, Hartmann JT. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. European Organisation and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. Lancet Oncol. 2014; 4: 415-423.
- Jensen SA, Vilmar A, Sørensen JB. Adjuvant chemotherapy in elderly patients (≥75yr) completely resected for colon cancer stage III compared to younger patients. Med Oncol. 2006; 4: 512–531.
- Serizawa I, Kagei K, Kamada T. Carbon ion radiotherapy for unresectable retroperitoneal sarcomas. Int J Radiat Oncol Biol Phys. 2009; 75: 1105- 1110.
- Paumier A, Le Péchoux C, Beaudré A. IMRT or conformal radiotherapy for adjuvant treatment of retroperitoneal sarcoma? RadiotherOncol. 2011; 99: 73-78.