Original Article
Cardiovascular Co-Morbidity and the Outcome after Myeloablative Hematopoietic Stem Cell Transplantation for Advanced Lymphoma
Hsueh Ju Lu1,5#, Ya-Ching Chen8#, Ming-Huang Chen2,6, Wei-Hsin Chen7, Jui-Hung Kao7, Chia-Ping Shen7, Feipei Lai7, Chi-Ying F. Huang9, Yu-Feng Hu3,4* and Peter Mu-Hsin Chang2,6*
1Department of Internal Medicine, Chung Shan Medical University Hospital, Taiwan
2Department of Oncology, Taipei Veterans General Hospital, Taiwan
3Division of Cardiology, Department of Medicine, Taipei Veterans General Hospital, Taiwan
4Department of Cardiovascular Research Institute, National Yang-Ming University, Taiwan
5Department of Medicine, Chung Shan Medical University, Taiwan
6Department of Medicine, National Yang-Ming University, Taiwan
7Department of Biomedical Electronics and Bioinformatics, National Taiwan University, Taiwan
8Department of Medical Research, Show Chwan Memorial Hospital, Taiwan
9Institute of Biopharmaceutical Sciences, National Yang-Ming University, Taiwan
#These authors contribute equally to this work.
*Corresponding author: Yu-Feng Hu, Department of Cardiology,
Taipei Veterans General Hospital, 201,
Sec. 2, Shih-Pai Road, Taipei, Taiwan
Peter Mu-Hsin Chang, Department
of Medical Oncology, Department of
Oncology, Taipei Veterans General
Hospital, Taipei, Taiwan. 201, Sec. 2,
Shih-Pai Road, Taipei, Taiwan
Published: 24 Sep, 2016
Cite this article as: Lu HJ, Chen Y-C, Chen M-H,
Chen W-H, Kao J-H, Shen
C-P, et al. Cardiovascular Co-
Morbidity and the Outcome after
Myeloablative Hematopoietic Stem
Cell Transplantation for Advanced
Lymphoma. Clin Oncol. 2016; 1: 1101.
Abstract
We evaluated cardiovascular-associated co-morbidities systematically in patients undergoing
hematopoietic stem cell transplantation (HCT) for lymphoma. In this study, 101 lymphoma patients
(41.3 ± 13.6 years) receiving salvage myeloablative HCT with high-dose conditioning treatments
were recruited. Cardiovascular associated factors were reviewed and correlated to outcome after
HCT. The mean follow-up period was 1074.8 ± 739.2 days. The Overall Survival (OS) rate was
68.3% and the 3-month survival rate was 89.1%. Multivariate analysis revealed that the independent
risk factors for OS were High Sensitivity C-Reactive Protein (HsCRP), Abnormal Regional Wall
Motion (ARWM), age, and Eastern Cooperative Oncology Group (ECOG) performance status.
Combination of the 4 risk factors significantly increased the predictive value for OS. High HsCRP
levels were associated with higher cumulative tumor-related mortality (P=0.045). ARWM was
associated with both higher cumulative tumor-related mortality (P=0.01) and non-relapse mortality
(P=0.027). In conclusions, ARWM and HsCRP were important predictors for long-term survival in
lymphoma patients undergoing HCT.
Keywords: Abnormal regional wall motion; High sensitivity c-reactive Protein; Hematopoietic stem cell transplantation
Introduction
Non-Hodgkin Lymphomas (NHLs) and Hodgkin Disease (HD) are ranked among the top
10 cancer causes of death all over the world [1]. Currently, the treatment option with the highest rate of long term survival in refractory and advanced lymphoma is hematopoietic stem cell transplantation (HCT). The effects of previous cumulative anthracycline doses [2,3] and high-dose cyclophosphamide, especially in patients who receive mediastinal radiation during HCT [4,5], result in a higher risk of cardiovascular complications [3,6]. Lymphoma survivors frequently experience cardiovascular events during long term follow-up [2-8]. Childhood cancer survivor studies have shown that patients with HD commonly develop serious cardiovascular conditions during long
term follow-up [2]. The heart is one of the most frequently affected organs in patients who have
received allogeneic HCT as treatment for lymphoma or chronic lymphocytic leukemia [9]. Patients
with impaired cardiac function show significantly increased treatment-related mortality, which may
offset the potential clinical benefit of the transplant [5]. Although several studies have investigated the cardiovascular-associated risk factors after HCT, none of those studies were limited to lymphoma patients who have undergone intensive treatments. In addition, data on the usefulness of eligibility criteria associated with cardiac function in lymphoma patients who are undergoing HCT are also sparse.
Some important studies have shown that the HCT-specific
comorbidity index (HCT-CI), which combines the risk factors derived
from different organ systems, can successfully predict Overall Survival
(OS) after HCT [4,10]. There are some cardiovascular-associated
risk factors such as hyperlipidemia, and Abnormal Regional Wall
Motion (ARWM) not involved in the HCT-CI. Several biomarkers
with prognostic value in patients with malignancy, such as troponin
and High Sensitivity C-Reactive Protein (HSCRP) [11-14], have not
been studied specifically in lymphoma patients receiving HCT. It
remains unclear if a detailed analysis of cardiovascular-associated risk
factors would lead to a more precise prognosis in lymphoma patients
receiving HCT.
The aims of the present study were to investigate possible
cardiovascular and non cardiovascular-associated risk factors of long
term survival after HCT and to study the impact of cardiovascular
characteristics, namely ARWM, Hs CRP and troponin levels, on
prognosis.
Methods
Patient selection
Patients with lymphoma who received myeloablative HCT
(75 autologous, 26 allogeneic) during the period January 2003
to November 2008 at the Taipei Veterans General Hospital were
retrospectively analyzed. A total of 101 patients were included in the
study (NHLs, n = 86; HD, n = 15). The retrospective review included
the assessment of cardiovascular-associated risk factors such as
diabetes mellitus, hypertension, and hyperlipidemia. All patients
received a comprehensive evaluation of heart function, including
resting ejection fraction by first pass and wall motion studies by 99mTcpertechnetate
before HCT. The comorbidity scores were assigned by
the single principal evaluator using the HCT-CI after reviewing the
medical records and laboratory values of all patients. Ethical approval
was granted by the Institutional Review Board of the Veterans General
Hospital, Taipei, Taiwan (VGHIRB No. 201004034IC).
Chemotherapy before stem cell transplantation
First-line chemotherapy comprised R-CHOP (rituximab,
cyclophosphamide, doxorubicin, vincristine, prednisone) for
patients with Diffuse Large B Cell Lymphoma (DLBCL) and ABVD
(doxorubicin, bleomycin, vinblastine, dacarbazine) for patients with
HD. Second-line regimens included ESHAP (etoposide, prednisone,
cytarabine, cisplatin) or ICE (ifosfamide, carboplatin, etoposide),
followed by high-dose conditioning chemotherapy and HCT. The use
of HCT as salvage treatment was considered in patients who showed
an inadequate response to induction chemotherapy or experienced
relapse. The dose of stem cells for patients receiving autologous
HCT was greater than 2 × 106/kg. In patients who could not obtain a
sufficient self-stem cell dose and received allogeneic HCT, HLA typing
was performed using the polymerase chain reaction with sequence
specific primers. Among the patients who received allogeneic HCT,
23 had sibling donors and 3 had unrelated donors. All patients had 6
completely matched alleles compared with their donors, except for 1
patient who had 1 mismatched allele.
Conditioning regimen for stem cell transplantation
Most patients received conditioning regimens composed of
BEAM (carmustine, etoposide, cytarabine, melphalan) or BEAC
(carmustine, etoposide, cytarabine, cyclophosphamide). Cy TBI conditioning consisted of 120 mg/kg cyclophosphamide, followed
by total body irradiation with 12–13.2 Gy in 3 patients with
highly aggressive lymphoma who accepted allogeneic HCT. The
immunosuppressive regimen after allogeneic HCT included the
following: a dose of 15 mg/m2 of MTX intravenously administered
on day 1 and 10 mg/m2 on days 3, 6, and 11. The administration of
CsA (1.5 mg/kg intravenously or 6.25 mg/kg orally every 12 hours)
was started 1 day before marrow infusion and continued until day 50
after HCT. The dose was then decreased by 5% weekly until 6 months
after transplantation if the patients were devoid of manifestations
of acute or chronic Graft Versus Host Disease (GVHD). For patients
with GVHD, azathioprine and steroids were added to the treatment
regimen until the disease was controlled.
Cardiovascular biomarkers and clinical events
Hs CRP levels were determined using particle-enhanced
immunoturbidimetry with latex micro particles sensitized with
duck anti-CRP immunoglobulin Y (Good Biotech Corp, Taichung,
Taiwan). Troponin I was detected by the ADVIA Centaur cTnI
chemiluminescence assay on an ADVIA Centaur Analyzer (Siemens
Medical Solutions Diagnostics, Tarrytown, NY, USA). Before HCT,
peripheral blood samples were collected from each patient and stored
in the blood bank at −80°C until use. Congestive Heart Failure (CHF)
was defined in patients who had at least one episode of shortness of
breath on minimal exertion or at rest (New York Heart Association
[NYHA] functional class III or IV) or paroxysmal nocturnal dyspnea
within the month before admission; and in patients with radiographic
evidence of cardiomegaly and acute pulmonary edema.
Disease status evaluation
Patients were staged based on the results of Computed
Tomography (CT) scanning and/or Positron Emission Tomography
(PET) scanning, and bone marrow biopsy was performed as indicated
by physicians. Treatment responses were evaluated using CT in most
cases and PET in some cases after 2005. Complete Response (CR),
Partial Response (PR), Stationary Disease (SD), and Progressive
Disease (PD) were defined according to RECIST criteria [15]. OS was
measured from the time of HCT to the date of death from any cause
or the last follow-up. Non-Relapse Mortality (NRM) was determined
by physicians when the cause of death was independent of lymphoma
disease and without recurrence.
Prognostic predictors and survival analysis
All the possible predictive factors were subjected to univariate
and Cox proportional hazards regression analysis (adjusted for
all univariate factors found to have P value <0.05) to find the
independent predictors of survival. The Kaplan-Meier method was
used to estimate the OS rate. The survival of patients with different
prognostic factors was compared using the log rank test. With the
exception of OS and 3-month survival, comparisons of continuous
data were performed using the Student’s t-test, and The comparisons
of categorical data were performed using a Chi-square test with a
Yates’ correction or Fisher’s exact test, such as the relationships
among cumulative mortality, Hs CRP, and ARWM. P values less than
0.05 were considered statistically significant. Statistical analyses were
performed with the statistical package SPSS for Windows (Version
17.0, SPSS Inc, Chicago, IL).
Results
Baseline characteristics
The characteristics of the 101 patients included in the present study are shown in Table 1. The mean age was 41.3 ± 13.6 years, and the mean follow-up period was 1074.8 ± 739.2 days (range: 14–2392 days). The patients were predominantly male (59.4%). NHLs was the
definitive diagnosis in the majority of patients (85.1%). Most patients
had Eastern Cooperative Oncology Group (ECOG) performance
scores of 0 or 1 and no HCT-CI comorbidities (70.3%). The mean
Hs CRP level was 0.96 ± 2.76 mg/dL and the mean troponin I level
was 0.05 ± 0.21ng/mL. Seven patients had a Left Ventricular Ejection
Fraction (LVEF) lower than 45% before HCT. The overall incidence of
ARWM was 47.5%. The OS rate was 68.3% and the 3-month survival
rate was 89.1%.
Cardiovascular events and non-relapse mortality
Total cardiovascular events in 21 patients with CHF and in 3
patients with major cardiovascular events after HCT were reviewed. As
shown in Table 2, there were 3 major cardiovascular events (1 sudden
cardiac death with an unknown cause, 1 ventricular tachycardia, 1
myocardial infarction), while the 2nd and 3rd patients were associated
with lymphoma progression. The mortality rate was higher among
patients with cardiovascular events than among patients without
cardiovascular events (57.1% vs. 25.0%, P=0.01, data not shown).
Non-relapse mortality (NRM) was noted in 14 patients (13.9%). The
causes of NRM included sepsis (n=7), fulminant hepatitis (n=3),
GVHD (n=1), adult respiratory distress syndrome (n=1), thrombotic
thrombocytopenic purpura (n=1), and sudden cardiac death (n=1).
Long term prognostic factors after HCT
Univariate analysis showed that age, disease status, performance
status, ARWM, Hs CRP have significantly predictive values
(Table 3). After Cox regression analysis with all predictive factors,
only ARWM, Hs CRP, age, and ECOG performance status were
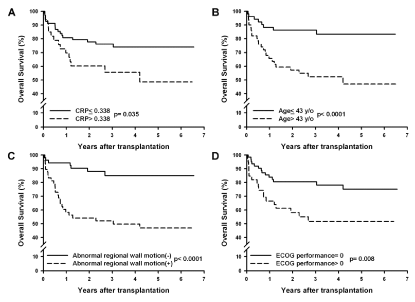
independent risk factors of long term OS. Kaplan-Meier survival
curves for OS were constructed according to the 4 independent risk
factors (Figure 1), with cut-off values determined by individual ROC
curves (Hs CRP >0.338 mg/dL, sensitivity: 46.9%, specificity: 73.9%,
AUC: 0.60; age >43 years, sensitivity: 75.0%, specificity: 62.3%, AUC:
0.68; performance >0, sensitivity: 56.2%, specificity: 69.6%, AUC:
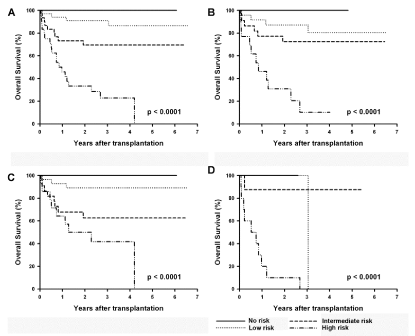
0.65). Combining the prognostic factors to categorize no, one, two,
and equal or more than 3 risk factors groups revealed that survival
progressively decreased as the number of risk factors increased
(Figure 2A). The categorization could also be applied to patients who
do not have risk factors traditionally associated with the HCT-CI
index (n=71) (Figure 2B).
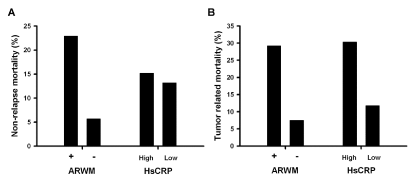
Relationship among cumulative mortality, Hs CRP and ARWM
The relationships among the cumulative mortalities, Hs CRP
levels, and ARWM were further investigated. High Hs CRP levels
(>0.338 mg/dL) were associated with higher cumulative tumorrelated
mortality (P=0.045) but not with non-relapse mortality
(P=0.99). However, ARWM was associated with both tumor-related
mortality (P=0.01) and non-relapse mortality (P=0.027) (Figure 3).
Table 1
Discussion
The present study is the first to show that ARWM, Hs CRP, age,
and ECOG performance are independent predictors of long term OS
in patients with lymphoma after HCT. Furthermore, ARWM was
associated with both cumulative NRM and tumor-related mortality
and HsCRP was specifically associated with cumulative tumorrelated
mortality.
Long term survivors of allogeneic HCT as well as patients
with lymphoma are at increased risk of premature cardiovascular accidents [2-8]. However, it remains unclear whether these
cardiovascular events are associated with differences in survival.
Although cardiovascular events occurred frequently after HCT
in this study, multivariate Cox regression analysis revealed that
those events were not independently associated with OS. This
finding could be attributed to the advancement in the treatment of
cardiovascular diseases in recent years, especially in cases of heart
failure, which has significantly reduced the mortality of patients with
cardiovascular complications [18], while most of the mortality is due
to tumor progression or infection events. The collinearity between
comorbidities and cardiovascular events might also lead to a lack of
significance after multivariate analysis.
Screening of all cardiovascular associated risk factors and
characteristics showed that ARWM and HsCRP were independently
associated with OS. HsCRP, which has been associated with a high risk
of coronary artery disease, arrhythmia, and cardiovascular mortality, is
considered an important cardiovascular associated risk factor [19,20].
Recently, HsCRP has also been reported to be a predictor of long term
mortality in patients with lymphoma after chemotherapy, chronic
myeloid leukemia after HCT, or early relapse after chemotherapy
[12,13]. Microscopy data from human lymphoma tissues revealed that tumor-associated macrophages, which are associated with
inflammatory status, were correlated with a shortened progressionfree
survival and relapse after autologous HCT [21]. The present
study showed that HsCRP was associated with higher cumulative
tumor-related mortality but not with NRM. These findings suggest
that HsCRP might be the manifestation of inflammatory infiltration
in patients with lymphoma, rather than playing a role in the incidence
of cardiovascular events. Acute cardiovascular dysfunction induced
by acute illness and severe sepsis is associated with increased mortality
[22]. In patients undergoing HCT, cardiovascular decomposition
induced by either tumor progression or sepsis might contribute to
higher mortality rates and be masked by the dominant manifestations
of sepsis or tumor progression. Although apparent LVEF dysfunction
has been correlated with mortality in patients after HCT [10,23].
However, in patients with normal resting LVEF, cardiovascular
reserve in response to stress such as exercise, or sepsis may be a better
indicator of mortality. ARWM, which is associated with cardiac
dyssynchrony, is an early feature of heart failure in patients with
preserved LVEF [24,25], and might be associated with stress-induced
cardiac dysfunction [26]. Therefore, ARWM, as an early predictor
of subclinical cardiovascular dysfunction, might be exacerbated and be associated with mortality under stress conditions such as tumor progression or severe infection.
HCT-CI is an important risk assessment score in lymphoma patients receiving HCT [4,27]. In the present study, HCT-CI was
not associated with prognosis. Compared to the findings reported
by Farina et al., the population in the present study was younger
(median age: 43 years vs. 53 years), and had lower HCT-CI scores (patients with HCT-CI = 0, 70.3% vs. 32% in the study by Farina et al. [4]. Similar differences were found between the present study and
the findings reported by Plattel et al. [25] (age, 43 years vs. 52 years;
patients with HCT-CI = 0, 70.3% vs. 52%) [27]. The younger age and
lower HCT-CI scores in the present study population might explain
why HCT-CI did not have the same predictive value as in previous
studies. The present study introduced new cardiovascular associated
risk factors, which might help in the stratification of patients with
low HCT-CI scores or those without comorbidities (Figure 2B). There
are limitations in this retrospective analysis, including the inevitable
selection bias from a single center and small number of studied
populations. However, the high-risk group of patients with 3 or more
risk factors had a very poor prognosis, which may discourage the
use of HCT as treatment for lymphoma in that patient population
in the future. A larger scale study is necessary to further validate the
present findings. In conclusion, ARWM is a cardiovascular associated
factor for long term OS in lymphoma patients after HCT. HsCRP is
specifically correlated with tumor-related mortality. Patients with
equal or more than 3 independent risk factors may be discouraged for
HCT in the future clinical practice.
Table 2
Table 3
Figure 1
Figure 1
Kaplan-Meier survival curves of 4 independent prognostic factors of overall survival. (A) Lower serum HsCRP level (cut-off value, 0.338 mg/dL;
P< 0.035), (B) younger age while receiving transplantation (cut-off value, 43 years; P< 0.0001), (C) normal regional wall motion (P< 0.0001), and (D) ECOG
performance=0 (P< 0.008) are independent predictors of overall survival.
Figure 2
Figure 2
Kaplan-Meier survival curves of the risk groups according to the categorization.
Each of the 4 independent prognostic factors (HsCRP, age, ARWM, and ECOG performance) was viewed as 1 risk factor and then added together. (A) In the
whole study population, there were 4 groups of lymphoma patients undergoing stem cell transplantation based on the risk factors=0 (n=13), 1 (n=34), 2 (n=30),
equal or more than 3 (n=24). (P< 0.0001); (B) in the subgroup without HCT-CI comorbidities, there were still significant survival differences between the 4 risk
groups (P< 0.0001).
Figure 3
Figure 3
Comparison of tumor related vs. non-relapse mortality between HsCRP and ARWM. (A) The ARWM (+) patients had higher non-relapse mortality rates
than the ARWM (−) patients (P=0.027), while there were no differences in HsCRP levels (P=0.99). (B) Both ARWM (+) and high HsCRP were correlated with higher
tumor-related mortality (P=0.01 and 0.045, respectively).
Acknowledgement
We thank all the hematologists and members of the VGHTPE multi-modality lymphoma treatment team for taking care of the patients in this study. This study was partially supported by the Taiwan Clinical Oncology Research Foundation. This work was also supported by research grants from the Taipei Veterans General Hospital (V97C1-059, V97A-097, V98C1-037, and V99C1-120), Show Chwan Memorial Hospital (RD105021), and the National Scientific Council (NSC97-2314-B-010-037-MY3, NSC97-2314-B-010-038, NSC98-2314-B-010-031-MY3).
References
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics. 2008; 58: 71-96.
- Castellino SM, Geiger AM, Mertens AC, Leisenring WM, Tooze JA, Goodman P, et al. Morbidity and mortality in long-term survivors of Hodgkin lymphoma: a report from the Childhood Cancer Survivor Study. Blood. 2011; 117: 1806-1816.
- Peres E, Levine JE, Khaled YA, Ibrahim RB, Braun TM, Krijanovski OI, et al. Cardiac complications in patients undergoing a reduced-intensity conditioning hematopoietic stem cell transplantation. Bone Marrow Transplant. 2010; 45: 149-152.
- Farina L, Bruno B, Patriarca F, et al. The hematopoietic cell transplantation comorbidity index (HCT-CI) predicts clinical outcomes in lymphoma and myeloma patients after reduced-intensity or non-myeloablative allogeneic stem cell transplantation. Leukemia. 2009; 23: 1131-1138.
- Fujimaki K1, Maruta A, Yoshida M, Sakai R, Tanabe J, Koharazawa H, et al. Severe cardiac toxicity in hematological stem cell transplantation: predictive value of reduced left ventricular ejection fraction. Bone Marrow Transplant. 2001; 27: 307-310.
- Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009; 53: 2231-2247.
- Baker KS, Ness KK, Steinberger J, Carter A, Francisco L, Burns LJ, et al. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: a report from the bone marrow transplantation survivor study. Blood. 2007; 109: 1765-1772.
- Tichelli A, Passweg J, Wójcik D, Rovó A, Harousseau JL, Masszi T, et al. EBMT Late Effects Working Party.et al. Late cardiovascular events after allogeneic hematopoietic stem cell transplantation: a retrospective multicenter study of the Late Effects Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2008; 93: 1203-1210.
- Sorror ML, Storer BE, Maloney DG, Sandmaier BM, Martin PJ, Storb R. Outcomes after allogeneic hematopoietic cell transplantation with nonmyeloablative or myeloablative conditioning regimens for treatment of lymphoma and chronic lymphocytic leukemia. Blood. 2008; 111: 446-452.
- Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005; 106: 2912-2919.
- Cardinale D, Sandri MT. Role of biomarkers in chemotherapy-induced cardiotoxicity. Prog Cardiovasc Dis. 2010; 53: 121-129.
- Pavlů J, Kew AK, Taylor-Roberts B, Auner HW, Marin D, Olavarria E, et al. Optimizing patient selection for myeloablative allogeneic hematopoietic cell transplantation in chronic myeloid leukemia in chronic phase. Blood. 2010; 115: 4018-4020.
- Herishanu Y, Perry C, Braunstein R, Metser U, Goor O, Rogowski O, et al. Early-mid treatment C-reactive protein level is a prognostic factor in aggressive non-Hodgkin's lymphoma. Eur J Haematol. 2007; 79: 150-154.
- Cardinale D, Sandri MT, Colombo A, Colombo N, Boeri M, Lamantia G, et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation. 2004; 109: 2749-2754.
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000; 92: 205-216.
- Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009; 53: e1-e90.
- Musunuru K, Kral BG, Blumenthal RS, Fuster V, Campbell CY, Gluckman TJ, et al. The use of high-sensitivity assays for C-reactive protein in clinical practice. Nat Clin Pract Cardiovasc Med. 2008; 5: 621-635.
- Masson S, Aleksova A, Favero C, Staszewsky L, Bernardinangeli M, Belvito C, et al. Predicting atrial fibrillation recurrence with circulating inflammatory markers in patients in sinus rhythm at high risk for atrial fibrillation: data from the GISSI atrial fibrillation trial. Heart. 2010; 96: 1909-1914.
- Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, et al. Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. N Engl J Med. 2010; 362: 875-885.
- Zanotti-Cavazzoni SL, Hollenberg SM. Cardiac dysfunction in severe sepsis and septic shock. Curr Opin Crit Care. 2009; 15: 392-397.
- Zangari M, Henzlova MJ, Ahmad S, Scigliano E, Isola L, Platnik J, et al. Predictive value of left ventricular ejection fraction in stem cell transplantation. Bone Marrow Transplant. 1999; 23: 917-920.
- Yamamoto A, Takahashi N, Abe K, Kobayashi Y, Tamai J, Munakata K. Regional left-ventricular diastolic wall motion assessed by a new program for ECG-gated myocardial perfusion SPECT in early-stage heart failure. J Nucl Cardiol. 2008; 15: 375-382.
- Yamamoto A, Takahashi N, Ishikawa M, Abe K, Kobayashi Y, Tamai J, et al. Relationship between left ventricular function and wall motion synchrony in heart failure assessed by ECG-gated myocardial perfusion SPECT. Ann Nucl Med. 2008; 22: 751-759.
- Okeie K, Shimizu M, Yoshio H, Ino H, Yamaguchi M, Matsuyama T, et al. Left ventricular systolic dysfunction during exercise and dobutamine stress in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2000; 36: 856-863.
- Plattel WJ, Kluin-Nelemans HC, de Bock GH, van Imhoff GW. Prognostic value of comorbidity for auto-SCT eligibility and outcome in relapsed or refractory aggressive non-Hodgkin's lymphoma. Bone Marrow Transplant. 2011; 46: 827-834.