Review Article
Robotic-Assisted Partial Cystectomy and Segmental Ureterectomy: Analysis of Efficacy and Oncologic Outcomes
Shen Yifan*, Xu Tianyuan, Chen Shanwen and Shen Zhoujun
Department of Urology, Huashan Hospital, China
*Corresponding author: Shen Yifan, Department of Urology, Huashan Hospital, 12 Middle Wulumuqi Road, Shanghai, China
Published: 16 Sep, 2016
Cite this article as: Yifan S, Tianyuan X, Shanwen
C, Zhoujun S. Robotic-Assisted
Partial Cystectomy and Segmental
Ureterectomy: Analysis of Efficacy and
Oncologic Outcomes. Clin Oncol. 2016;
1: 1098.
Abstract
Objective: To report on patients undergoing Robot-Assisted Partial Cystectomy (RAPC), and
segmental ureterectomy focusing on the operational efficacy and Oncologic Outcomes over a range
of clinical, anatomical and pathological variables, as well as the overall oncological efficacy of these
organ-sparing approach.
Methods and Patients: We retrospectively examined the robotic surgical database at Ruijin hospital
and Huashan hospital to isolate cases of urologic malignancy managed using robotic technology
from 2009 to 2016. During this period, 10 patients with biopsy-confirmed urothelial carcinoma of
the bladder (cT1-4N0M0) were treated with RAPC plus chemotherapy and/or radiation therapy.
And 6 patients with urothelial carcinoma of the ureter (cT0-2N0M0) were treated with RASU plus
chemotherapy and/or radiation therapy.
Results: RAPC was performed in 10 patients and the mean total operative time including
Cystoscopy was 126.36minutes (90-180), mean estimated blood loss was 95.45mL (50-150). There
were no intraoperative complications. The mean postoperative length of stay was 16.54 days (9-42).
One patient suffered urine leak, who finally required a secondary operation at 2 weeks after RAPC.
2 patients developed cancer recurrence in the first year after RAPC. For the 6 patient underwent
RASU, the mean operative time was 106.67minutes (90-160), and the estimated blood loss was
83.33mL (50-200). Meanwhile, there were no intraoperative complications. The mean postoperative
length of stay was 9.67 days (8-15). And the follow-up for these patients showed no recurrence at
the 12 months.
Conclusion: RAPC and RASU confer the ability to achieve favorable outcomes. Robotic-assisted
organ-sparing surgery should be considered a valid and meaningful option for the patients of
bladder and ureter malignancy. Patient selection and accurate risk estimation are important, which
immediately affect the oncological outcomes. More practice should be done, especiallyour data of
follow-up, which is our major limitation of the study.
Keywords: Urethial cancer; Partial cystectomy; Segmental ureterectomy; Robot-assisted; Complications; Outcomes
Introduction
Since the first reported robotic-assisted laparoscopic prostatectomy took place in Paris in 2000,
there has been a rapid adoption of robotic procedure in urology and another surgical subject [1,2].
The advantages of robotic surgery system whose wide application in the surgery of bladder, prostate,
kidney and ureter, include magnified 3D high definition surgical view, improved dexterity with
articulating Endo Wrist® instruments with 7 of freedom, etc [3]. Those unique superiorities not only
improve the surgical outcome, but also let us review the present operation methods [4].
Organ-sparing surgery in urologic malignancy treatment once has been not highly appraised for
its incomplete dissection which may lead to inevitably high risk of recurrence [5-7]. However, with
the assistance of robotic surgery, the clinical efficiency of some organ-sparing surgeries such as partial
cystectomy and segmental ureterectomy have been reassessed. Partial cystectomy, a comparably
shorter, less morbid surgery without the need for urinary diversion, has been represented an option
for management of bladder cancer for selected patients according to a series of researches in the past
decade [4]. Segmental Ureterectomy (SU) is another procedure in treatment of ureter carcinoma
which could keep the kidney for avoiding the Chronic Kidney Disease (CKD) after the surgery
[8]. Several evidences show similar oncologic outcomes between
traditional nephrouretrectomy (NU) and SU [9].
In this study, we report on our experience with robotic partial
cystectomy and segmental ureterectomy, with a focus on perioperative
outcomes over a wide range of clinical, anatomical and pathological
variables.
Table 1
Table 2
Methods and Patients
We retrospectively examined the robotic surgical database at
Ruijin hospital and Huashan hospital to isolate cases of urologic
malignancy managed using robotic-technology from 2009 to 2016.
During this period, 10 patients with TUR biopsy-confirmed urothelial
carcinoma of the bladder (cT1-4N0M0) were treated with RAPC
plus chemotherapy and/or radiation therapy. And 6 patients with
urothelial carcinoma of the ureter (cT0-2N0M0) were treated with
RASU. The detailed inclusion criteria we mentioned in the section
Discussion.
All robot-assisted surgeries were performed at these two medical
institutions by our senior author (Zhoujun Shen) using the da Vinci
SI system (Intuitive Surgical, Mountain View, CA, USA). Before the
surgery, all patients underwent radiological examine, cytological
assessment, anesthesia evaluation, and cystoscopy. Random bladder
biopsies performed during TUR for the patients with bladder
cancer. The pathology was reviewed by a dedicated genitourinary
pathologist. All perioperativecomplications occurring at≤ 90 days of
surgery we rerecorded and classified according to the Clavien–Dindo
classification of surgical complications [10]. Meanwhile, patients
were fully informed about the advantages and disadvantages of
organ-sparing therapy, and ultimately selected the final procedure.
The treatment protocol was approved by the ethics committee of the
institutions.
For RAPC, the main operation sequences were listed as
the following: 1. Place the ports for pneumoperitoneum, and a
Cystoscopy as a guide for a monopolar laparoscopic scissor to
delineate the necessary area of resection on the outside of the
bladder. Remove the cystoscope, place aurethral catheter, and dock
the robot. 2. The bladder is left attached to the anterior abdominal
wall and then carefully opened, avoiding direct manipulation of
the tumor. 3. Excising the tumor en-bloc with the tumor giving a
2-cm circumferential margin for adequate resection. 4. A distal
ureterectomy and ureteric re-implantation if tumor is 2 cm lateral to
the ureteral orifice and a frozen section is sent. 5. Place the specimen
in a bag and removed. 6. The bladder defect is closed and interrupted
double-layer closure, and PLND is performed.
For RASU, the main steps include: 1. Port placement according to
the site of the ureteric lesions. 2. Reflection of colon and exposure of
the retro peritoneum. 3. Ureteralmobilization. 4. Application of Hemo-
lock clips proximally and distally. 5. Excision of ureteral tumor
and frozen sections were routinely sent to assess appropriateness
of surgical margins. 6. Intracorporeal Double-J stent placement. 7.
ureteral–ureteral anastomosis, and PLND is performed.
Patients would then follow-up routinely for a standard oncologic
and functional surveillance protocol to evaluate for recurrence of
malignancy as follows: Standard laboratory basic metabolic panel
analysis postoperatively at follow-up clinic visits, CT urography
performed approximately 12 weeks after surgery, and cystoscopy at
6 months postoperatively.
Results
The clinical and pathological characteristics of patients treated
with RAPC and RASU are summarized in the Table 1 and 2,
respectively.
RAPC was performed in 10 patients, including 8 men and 2
women, whose mean age was 65y (47-80). Before the RAPC, 9 patients
received cisplatin-based neo-adjuvant chemotherapy. The mean
total operative time including Cystoscopy was 126.36 minutes (90-
180), mean estimated blood loss was 95.45mL (50-150). There were
no intraoperative complications or conversion to pure laparoscopic
or open surgery, and no patient received a blood transfusion. The
final pathology after RAPC, 4 patients were T1 while 6 patients with
MIBC, 2 of which were pT3a. 9 patients had high grade tumor cells
both in TUR pathology and RAPC pathology. One patient with TUR
confirmed T1 and low grade had T2a and high grade lesion found
in the RAPC pathology. All patients had less than 3 tumors and
the mean tumor size was 2.71cm (1.0-8.0). All patients underwent
PLND during RAPC, and median of 8.9 (4-14) nodes were sent to
pathologist. One of these patients had nodal involvement. All patients
had a negative surgical margin and no CIS found in the RAPC
pathology. One patient suspected ureter involvement and underwent
ureteric re-implantation.
The mean postoperative length of stay was 16.54 days (9-42). No
patients had UTI or wound infection <90days, but one urine leak,
who finally required a secondary operation at 2 weeks after RAPC.
Adjuvant chemotherapy/radiotherapy protocol differed among the
patients for their own condition. One patient who had reoperation
history received radiotherapy and another patient received both
adjuvant chemotherapy and radiotherapy. 2 patients developed
cancer recurrence in the first year after RAPC. One underwent open
RC 6 months after RAPC and another one was performed TUR 8
months for bladder local recurrence.
For the 6 patient underwent RASU (Figure 1), the mean age
was 72y (48-84), and all were men. The mean operative time was
106.67 minutes (90-160), and the estimated blood loss was 83.33mL
(50-200). Meanwhile, there were no intraoperative complications
or conversion to pure laparoscopic or open surgery, and no patient
received a blood transfusion. 3 patients were T1 (50%) and the rest
were T2 according to the pathology after RASU. 4 patients in this
series received PLND. At last, none of patients had lymph nodes
involvement. All patients had 1 tumor and the mean tumor size was
1.5cm (1.0-8.0). All patients had a negative surgical margin and no
patients underwent ureteric re-implantation.
The mean postoperative length of stay was 9.67days (8-15). No
patients had UTI or wound infection <90days, but one postoperative
anemia, who received plasma transfusion twice at the 6th and 7th day
after RASU. All the patients received adjuvant radiotherapy and 3
received cisplatin-based chemotherapy after the surgery and the
follow-up for these patients showed no recurrence at the 12 months.
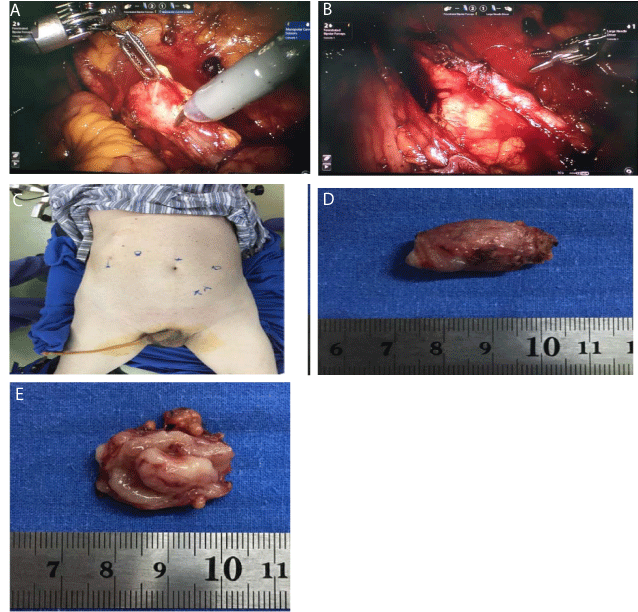
Figure 1
Figure 1
Robotic-assisted segmental ureterectomy (patient 5, right side). A. Ureteral lesion (during RASU); B. Ureteral–ureteral anastomosis; C. Port placement;
D. Ureteral specimen; E. Ureteral specimen (open).
Discussion
Laparoscopic procedure has become the important technique dealing with urologic disease for its minimal-invasive advantages which gain recognition among the surgeons and patients in the past decades [11]. During this period, laparoscopic technique, from the first laparoscopic radical prostatectomy in 1992 [12] to roboticassisted laparoscopic procedure nowadays, has been developing constantly. In addition, the novel surgical methods also promote the continuous improvement and perfection of the concept of urologists and this study mostly focus on the robotic organ-sparing approach.
RAPC
Radical cystectomy with PLND is the gold standard surgical
intervention for muscle-invasive bladder cancer [13], which needs
reconstruction with a urinary diversion for maintaining the normal
function of urination. Yu et al. [14] reported that patients undergoing
RARC compared with open RC had fewer inpatient complications
(49.1% vs. 63.8%, P=0.035) and fewer deaths (0% and 2.5%, P <0.001).
The high risk of complications promotes us searching a method for
not only achieving the satisfactory of oncological outcomes but also
minimal perioperativemorbidity and complications.
Partial Cystectomy (PC), once have been considered an
incomplete surgery for its high risk of recurrence [5,7,15], has shown
safety and oncological efficacy among properly selected patients with
the assistance of robotic system according to some of last studies.
There were several case reports in the past decades showing the
advantages of RAPC. Kim et al. [16] reported that 4 patients and
Allaparthi et al. [17] 3underwent RAPC, which started as an initial
attempt for the feasibility of RAPC. Up to now, David M. Golombos
et al. [18] identified 29 patients in 2015 who underwent RAPC. This
study showed 5-year overall and recurrence-free survival rates of
RAPC were 79% and 68%, respectively [18]. And they demonstrated
that RAPC could be an optimal approach for experienced surgeons
which would achieve favorable outcomes with low morbidity and
reduced hospital stays.
For PC, the selection criteria are critical important, and the
tradition data showed only highly-selected patients are suitable
for PC. In addition, consideration of the realistic condition that
the healthier patients are more likely to receive RC and finally PC
accounts for 7–10% of allcystectomies performed in USA [19,20].
In our series, we formulate our tactics as following: 1. No evidence
of CIS confirmed by TUR; 2. No involvement of the bladder neck
or urethra; 3. The patient’s option for bladder-sparing therapy. The
exclusion criteria included invasive tumors in the trig one and MIBC
with invasion of the prostate. Tumors that were within 2 cm lateral to
the ureteral orifice were not excluded from PC. cT2 and solitarycT3
MIBCs were strong candidates for PC.
As arobotic-assisted organ-sparing therapy, the advantages
for RAPC include low morbidity and complication rates.Inour10
patients’ series, the mean total operative time including Cystoscopy
was 126.36minutes (90-180) and mean estimated blood loss was
95.45mL (50-150). No intraoperative complications occurred and
no conversion to pure laparoscopic or open surgery. One patient
suffered from urine leak, who finally received a secondary operation
at 2weeks after RAPC. For hospital stay, RAPC appears to compare
quite favorably to reported open series [21], and higher-volume
institutions and surgeons yield better outcomes [22]. But the median
length of hospital stay in our series was 16 days, which seemed quite
different from the previous studies may due to the weak support from
community health service in our country.
The risk of recurrence of PC still is our focus of attention, which
once was seemed as the major limitation of PC, for it historically
recorded range between 40% and 78% [23]. In our short follow-up
period, two patients showed local recurrence and received operation
for treatment. More data would be displayed next then.
All patients underwent PLND during RAPC in this study, and
a median of 8.9 (4-14) nodes were sent to pathologist. One of these
patients had nodal involvement and this patient received both
adjuvant chemotherapy and radiotherapy. However, the data of
RAPC with PLND is still lacking. In the study of David M. Golombos,
they performed 90% of their patients and giving a relatively favorable
outcome. In the past, the underutilization of PLND during PC is as
low as 23%, which may be associated with the poor PC outcomes [24].
Although a more extensive lymphadenectomy may provide more
accurate pathologic staging and survival benefits, one must carefully
evaluate the risks associated with an extended lymph node dissection
[25].
RASU
Nephrouretrectomy (NU) with removal of bladder cuff is the
standard surgery for upper tract urothelial carcinoma [26]. However,
Nephrectomy is associated with a reduction of global renal function
[8]. And patients with impaired renal function would be ineligible to
receive cisplatin-based therapy, which may affect the recurrence of
the disease.
Segmental Ureterectomy (SU) is an another organ-sparing
procedure which used in treatment of ureter carcinoma. SU keeps
the kidney for avoiding the chronic kidney disease after the surgery
while it has traditionally been associated with high recurrence rates.
However, several evidences show recurrent rate has no differences
between NU and SU these years. Simonato et al. [9] showed their
results in 73 patients with pTa-T3 distal upper tract ureter carcinoma
with a 5-year RFS, OS and CSS rates of 82.2, 85.3 and 94.1%,
respectively. Jose A. Pedrosa et al. [27] reported are search of total 141
patients that localized recurrence occurred in 31.1% of SU/TU group
compared to 27.1% (p = 0.62) of the NU group in 2015. Their results
showed no significant survival between surgical approaches for upper
tract urothelial cancer.
The selection criteria for our RASU included these: a mid or
distal ureteral lesion/obstruction of theipsilateral renal moiety
on radiological data; low-grade, superficial pathological findings
underwent Cystoscopy and ureteroscopic biopsy; and no bladder
lesions. For the 6 patient selected for RASU, the mean age was 72y
(48-84), and all were men. All the patients with negative frozen
margin during RASU. During the follow-up in the first 12-months,
no patients show local recurrence.
Robotic-assisted approach can access the grade and stage more
accurately of this disease, which may be critical important for the
oncological outcomes of the patients. In our series, 3 patients were T1
(50%) and the rest were T2 according to the pathology after RASU.
4 patients in this series received PLND. At last, none of patients had
lymph nodes involvement.
Conclusion
RAPC and RASU confer the ability to achieve favorable outcomes. Robotic-assisted organ-sparing surgery should be considered a valid and meaningful option for the patients of bladder and ureter malignancy. Patient selection and accurate risk estimation are important, which immediately affect the oncological outcomes. More practice should be done, especially our data of follow-up, which is our major limitation of the study.
References
- Menon M, Shrivastava A, Tewari A, Sarle R, Hemal A, Peabody JO, et al. Laparoscopic and robot assisted radical prostatectomy: establishment of a structured program and preliminary analysis of outcomes. J Urol. 2002; 168: 945-949.
- Binder J, Kramer W, Robotically-assisted laparoscopic radical prostatectomy. BJU Int. 2001. 87: 408-410.
- Khosla A, AA Wagner. Robotic Surgery of the Kidney, Bladder, and Prostate. Surg Clin North Am. 2016; 96: 615-636.
- Knoedler J, Frank I. Organ-sparing surgery in urology: partial cystectomy. Curr Opin Urol. 2015; 25: 111-115.
- Schoborg TW, Sapolsky JL, Lewis CJ, Carcinoma of the bladder treated by segmental resection. J Urol. 1979; 122: 473-475.
- Anderström C, Johansson SL, Pettersson S, Wahlqvist L. Carcinoma of the ureter: a clinicopathologic study of 49 cases. J Urol. 1989; 142: 280-283.
- Resnick MI, VJ O'Conor. Segmental resection for carcinoma of the bladder: review of 102 patients. J Urol. 1973; 109: 1007-1010.
- Weight CJ, Larson BT, Fergany AF, Gao T, Lane BR, Campbell SC, et al. Nephrectomy induced chronic renal insufficiency is associated with increased risk of cardiovascular death and death from any cause in patients with localized cT1b renal masses. J Urol. 2010; 183: 1317-1323.
- Simonato A, Varca V, Gregori A, Benelli A, Ennas M, Lissiani A, et al. Elective segmental ureterectomy for transitional cell carcinoma of the ureter: long-term follow-up in a series of 73 patients. BJU Int. 2012; 110: E744-749.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004; 240: 205-213.
- Rassweiler JJ. Teber D. Advances in laparoscopic surgery in urology. Nat Rev Urol. 2016. 13: 387-399.
- Schuessler WW, Schulam PG, Clayman RV, Kavoussi LR. Laparoscopic radical prostatectomy: initial short-term experience. Urology. 1997. 50: 854-857.
- Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet. 2009; 374: 239-249.
- Yu HY, Hevelone ND, Lipsitz SR, Kowalczyk KJ, Nguyen PL, Choueiri TK, et al. Comparative analysis of outcomes and costs following open radical cystectomy versus robot-assisted laparoscopic radical cystectomy: results from the US Nationwide Inpatient Sample. Eur Urol. 2012; 61: 1239-1244.
- Novick AC, Stewart BH. Partial cystectomy in the treatment of primary and secondary carcinoma of the bladder. J Urol. 1976; 116: 570-574.
- Dae Keun Kim, Jae Won Lee, Sung Yul Park, Yong Tae Kim, Hae Young Park, Tchun Yong Lee, et al. Initial experience with robotic-assisted laparoscopic partial cystectomy in urachal diseases. Korean J Urol. 2010; 51: 318-322.
- Allaparthi S, Balaji RR. Robotic partial cystectomy for bladder cancer: a single-institutional pilot study. J Endourol. 2010; 24: 223-227.
- Golombos DM, O'Malley P, Lewicki P, Stone BV, Scherr DS. Robotic Assisted Partial Cystectomy (RAPC): perioperative outcomes and early oncologic efficacy. BJU Int. 2016.
- Gray PJ, Fedewa SA, Shipley WU, Efstathiou JA, Lin CC, Zietman AL, et al. Use of potentially curative therapies for muscle-invasive bladder cancer in the United States: results from the National Cancer Data Base. Eur Urol. 2013; 63: 823-829.
- Fedeli U, Fedewa SA, Ward EM. Treatment of muscle invasive bladder cancer: evidence from the National Cancer Database, 2003 to 2007. J Urol. 2011; 185: 72-78.
- Kates M, Gorin MA, Deibert CM, Pierorazio PM, Schoenberg MP, McKiernan JM, et al. In-hospital death and hospital-acquired complications among patients undergoing partial cystectomy for bladder cancer in the United States. Urol Oncol. 2014; 32: e9-14.
- Shabsigh A, Korets R, Vora KC, Brooks CM, Cronin AM, Savage C, et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol. 2009; 55: 164-174.
- Smaldone MC, Jacobs BL, Smaldone AM, Hrebinko RL Jr. Long-term results of selective partial cystectomy for invasive urothelial bladder carcinoma. Urology. 2008; 72: 613-616.
- Fahmy N, Aprikian A, Tanguay S, Mahmud SM, Al-Otaibi M, Jeyaganth S, et al. Practice patterns and recurrence after partial cystectomy for bladder cancer. World J Urol. 2010; 28: 419-423.
- Buscarini M, Josephson DY, Stein JP. Lymphadenectomy in bladder cancer: a review. Urol Int. 2007; 79: 191-199.
- Buscarini M, Josephson DY, Stein JP. Streem, Evolving management of upper-tract transitional-cell carcinoma at a tertiary-care center. J Endourol. 2002; 16: 483-487.
- Pedrosa JA, Masterson TA, Rice KR, Kaimakliotis HZ, Monn MF, Bihrle R, et al. Oncologic outcomes and prognostic impact of urothelial recurrences in patients undergoing segmental and total ureterectomy for upper tract urothelial carcinoma. Can Urol Assoc J. 2015; 9: E187-E192.