Case Report
Splenic Marginal Zone Lymphoma with Concurrent Myelofibrosis: A Case Report
Slonim LB1*, Geller M1, Donovan V1 and Harris J2
1Departments of Pathology, Winthrop-University Hospital, USA
2Departments of Hematotology/Oncology, Winthrop-University Hospital, USA
*Corresponding author: Liron Barnea Slonim, Department of Pathology, Winthrop University Hospital, 222 Station North Plaza, Suite 618, Mineola, NY 11501, USA
Published: 12 Sep, 2016
Cite this article as: Slonim LB, Geller M, Donovan V, Harris
J. Splenic Marginal Zone Lymphoma
with Concurrent Myelofibrosis: A Case
Report. Clin Oncol. 2016; 1: 1090.
Abstract
Myelofibrosis associated with lymphoid neoplasms is a rare occurrence with the exception of hairy
cell leukemia. Its incidence in splenic marginal zone lymphoma, as we report in this article, has only
been reported once in the past.
We report the case of a 62 year old male with splenic marginal zone lymphoma and concurrent
myelofibrosis. The diagnosis of splenic marginal zone lymphoma was achieved by a combination
of clinical, morphological, and immunohistochemical findings. Increased reticulin staining of the
bone marrow, cytopenias and macrocytes on peripheral blood smear supported the diagnosis of
myelofibrosis. Features of primary myelofibrosis and other myelodysplastic features including
associated genetic mutations were absent.
Further investigation of the association between splenic marginal zone lymphoma and concurrent
myelofibrosis may assist in future identification of its effect on disease course and overall prognosis,
possibly playing a role in risk stratification and personalized therapy.
Background
Increased fibrosis in the bone marrow is common in several myeloid malignancies but its incidence in lymphoid malignancies is less common. Myelofibrosis is frequently encountered in cases of hairy cell leukemia but is much less common in other lymphoid malignancies [1]. To our knowledge, only one case was reported in Japan, in which Splenic marginal zone lymphoma was complicated by myelofibrosis, associated with bone marrow involvement of lymphoma cells [2]. Lymphoid myelofibrosis represents a particular and rare entity in which medullary fibrosis associated with abnormal lymphoproliferation replaces normal hematopoiesis. Here we report a second case of lymphoid myelofibrosis associated with splenic marginal zone lymphoma in a 62 year old male.
Case Presentation
We report a case of a 62-year-old male, with splenic marginal zone lymphoma. He presented
with lymphocytosis, normocytic normochromic anemia and thrombocytopenia. Peripheral smear
showed smudge cells, teardrop red blood cells and elliptocytes.
Blood chemistry studies showed elevation of lactate dehydrogenase (LDH) and decreased
haptoglobin, but no hemoglobinuria.
There was neither hypergammaglobulinemia nor monoclonal gammopathy. There was
significant splenomegaly on imaging.
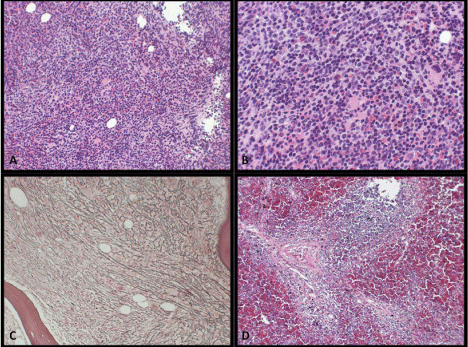
Bone marrow biopsy obtained from the left iliac crest showed a diffuse infiltrate of small mature
B cells (Figure 1) which were positive for CD20 and negative for CD5, CD10, BCL1, DBA44,
Annexin A1 and CD123. These findings were consistent with a low-grade B-cell lymphoproliferative
disorder. The marrow also showed foci of normal trilineage hematopoiesis with a spectrum of
maturing myeloid and erythroid precursors and normal appearing megakaryocytes. The bone
marrow also showed a diffuse moderate increase in marrow reticulin (MF-2) (Figure 1).
The patient's enlarged spleen was subsequently removed. The resected spleen weighed
2733g, and measured 30 x 17.5 x 8.5 cm. Pathology on the spleen showed a low grade lymphoma
involving the red and white pulp (Figure 1) with an immunophenotype similar to that seen in
the bone marrow, supporting a diagnosis of splenic marginal zone lymphoma. Extramedullary
hematopoiesis was identified. Reticulin fibrosis was not increased in
the spleen. Florescence in-situ hybridization analysis was negative for
rearrangements of BCL6 and MALT1 genes. However, three copies
of BCL6 gene signals were observed in 82.5% of the cells studied
suggesting the presence of trisomy 3 or gain of 3q in the sample. BCRABL1
fusion was negative. Analysis for the JAK2 V617F, JAK2 exon
12 and exon 13, MPL and calreticulin mutations was negative. Serum
TGF-beta were not elevated.
Figure 1
Figure 1
A, B. Bone marrow, H&E, medium and high power, infiltration of
the bone marrow by intertrabecular lymphoid aggregates composed of small
lymphoid cells. C. Bone marrow, reticulin stain, showing diffuse staining with
many intersections. D. Spleen, H&E, medium power, infiltration of white and
red pulp with small lymphocytes.
Discussion
In addition to primary and post chronic myeloproliferative
disorders, myelofibrosis has been reportedly associated with a large
subset of diseases. The association with lymphoproliferative diseases
other than hairy cell leukemia however, is rarely described. Rare cases
have been reported in multiple myeloma, T-cell lymphoma, and
lymphoplasmacytic cell lymphoma [3]. Only a single case has been
previously described with splenic marginal zone lymphoma [4].
A literature review from 2009 found an incidence of 6.6% of
myelofibrosis associated with lymphoma cases [5]; this was not
associated with a specific type of lymphoma, however there was a
relatively more frequent association of myelofibrosis with low-grade
non-Hodgkin lymphoma. B-symptoms and splenomegaly were
frequently present, and the LDH level was frequently elevated at
initial presentation. Two patients presented with myelofibrosis prior
to the diagnosis of lymphoma. Myelofibrosis was mild to moderate in
all cases. JAK2 V617F was negative in ten cases analyzed, suggesting
a distinct etiology from primary myelofibrosis. Response to therapy,
relapse rate, disease free and overall survival were not different from
lymphoma without myelofibrosis. A direct relation between the
tumor cell and myelofibrosis has also been evidenced by concomitant
regression of the myelofibrosis with the lymphoma in response to
chemotherapy and its reappearance with relapse.
Myelofibrosis has been suggested to arise in cytokines secreted by
the tumor cells and PDGF, TGF-beta, VEGF and beta-FGF have been
shown to play an important role in the development of secondary
stromal proliferation.
TGF-beta levels were reportedly elevated in the plasma of the
previously reported patient with splenic marginal zone lymphoma
and myelofibrosis, showing positive marrow immunostaining in the
lymphoma cell cytoplasm [6].
Interestingly, the spleen in that case also showed diffuse reticulin
fibrosis. A karyotype showed t (9;14)(p13;q32) and rearrangement of
the immunoglobulin JH gene by Southern blot was demonstrated.
Similarly, our case showed a low grade B-cell neoplasm with
B-symptoms, massive splenomegaly and elevated LDH. Anemia,
thrombocytopenia and leukoerythroblastic features were present.
The degree of myelofibrosis was similar and JAK2 V617F mutation
was negative, as were JAK2 exon 12 and exon 13, MPL and calreticulin
mutations, which are commonly seen in myelofibrosis associated
with myeloproliferative disorders. The negative BCR-ABL1 fusion
was negative, excluding myelofibrosis associated with CML.
TGF-beta serum levels were not elevated; however, other cytokines
secreted by tumor cells could be involved in the pathogenesis of
myelofibrosis associated with this case.
Treatment response remains to be seen in the patient presented
here. The small number of described cases makes it difficult to
assess whether myelofibrosis affects treatment response, however,
myelofibrosis can lead to significant morbidity, and further
investigations should be performed to allow identification of patients
at risk.
References
- Etienne A, Gruson B, Chatelain D, Garidi R, Royer B, Sevestre H,et al. Myelofibrosis-Associated Lymphoproliferative Disease: Retrospective Study of 16 Cases and Literature Review. Adv Hematol. 2009; 2009: 179847.
- Matsunaga T, Takemoto N, Miyajima N, Okuda T, Nagashima H, Sato T, et al. Splenic marginal zone lymphoma presenting as myelofibrosis associated with bone marrow involvement of lymphoma cells which secrete a large amount of TGF-β. Ann Hematol. 2004; 83: 322-325.
- Okabe S, Miyazawa K, Iguchi T.Peripheral T-cell lymphoma together with myelofibrosis with elevated plasma transforming growth factor-β1. Leuk Lymphoma. 2005; 46: 599-602.
- Riccardi A, Ucci G, Coci A.Bone marrow fibrosis in multiple myeloma. Am J Clin Path.1988; 90: 753-754.
- Rao SA, Gottesman SRS, Nguyen M C. T cell lymphoma associated with myelofibrosis. Leuk Lymphoma. 2003; 44: 715–718.
- Kimura A, Hyodo H, Nakata Y. Chronic lymphocytic leukemia associated with bone marrow fibrosis: possible role of interleukin 1 α in the pathogenesis. Am J Hematol. 1993; 43: 47–50.