Research Article
Outcome of Pediatric Patients with Low-Grade Astrocytoma of the Brain and Spinal Cord
Taneva M and Johnston DL*
Division of Pediatric Hematology/Oncology, Children’s Hospital of Eastern Ontario, Canada
*Corresponding author: Donna Johnston, Division of Hematology/Oncology, Children’s Hospital of Eastern Ontario, 401 Smyth Road, Ottawa, ON, Canada
Published: 09 Sep, 2016
Cite this article as: Taneva M, Johnston DL. Outcome
of Pediatric Patients with Low-Grade
Astrocytoma of the Brain and Spinal
Cord. Clin Oncol. 2016; 1: 1081.
Abstract
Background: Low-grade astrocytomas are the most common pediatric central nervous system
tumours. They can occur anywhere in the central nervous system and are often challenging to treat.
Methods: A retrospective review to compare the response to therapy for spinal cord astrocytomas
to that of brain astrocytomas.
Results: Five patients had low-grade spinal cord astrocytoma and 43 had low-grade brain
astrocytoma. One spinal cord and 16 brain patients underwent complete resection, and 2 and 20,
respectively, partial resection. Of the 56% total patients with a partial resection, 31.8% and 4.5%
received adjuvant chemotherapy and radiotherapy, respectively. 14% of all patients received
chemotherapy, without resection.
Three patients with spinal cord tumours underwent subtotal resection/biopsy followed by
chemotherapy. They showed a mean tumour decrease of 36.9%, and none relapsed. The remaining
patients showed tumour progression which responded to chemotherapy.
Ten brain tumour patients received chemotherapy and 4 received radiation. Tumour size decreased
by 44.5% with surgery/adjuvant therapy, and 27.1% with adjuvant therapy alone. Ten patients
relapsed at a mean of 9.6 months. Those treated underwent resection (n=3) or adjuvant therapy
(n=5) and 4 relapsed again.
Conclusions: All patients with low-grade spinal cord astrocytomas responded to chemotherapy and
none received radiation while 81.8% of those with brain astrocytoma responded to adjuvant therapy
including radiation.
Keywords: Low-grade astrocytoma; Chemotherapy; Therapy response
Introduction
Low-grade astrocytomas (LGA) are the most common central nervous system tumors in the
pediatric population [1]. They occur more frequently in the brain, representing 30-50% of newly
diagnosed brain neoplasms in this population [2], and approximately 60% of spinal cord tumors
[3,4]. The World Health Organization (WHO) classifies LGAs as Grade I or Grade II, and the most
common include pilocytic astrocytoma (Grade I), pilomyxoid astrocytoma (Grade II) and diffuse
astrocytoma (Grade II) [2].
Low-grade astrocytomas are often amenable to surgery. Gross total resection of LGAs has
been reported to offer 10-year survival rates greater than 90% [5,6], and to be the most important
prognostic factor for progression free survival [7-9]. Despite this, the management for LGAs in the
spinal cord remains controversial. Complete resection is often difficult due to the infiltrative nature
of these tumors [6], and may lead to increased morbidity due to pain, motor dysfunction and spinal
deformity [10].
The role of chemotherapy and radiation therapy for LGAs is also unclear. Protocols for therapy
of LGA include both Grade I and II astrocytomas so these were both examined for this study.
Retrospective studies have offered conflicting evidence on the value of adjuvant radiation on overall
survival and progression-free survival [6,11,12]. This, in addition to the known long-term neurologic
sequelae, warrants the study of safer methods. Adjuvant chemotherapy has been shown to be of
benefit in pediatric low-grade astrocytomas of the brain, with a response rate of 52-62% for both
newly diagnosed and progressing tumors [6], and has replaced radiation as first-line therapy when
resection is not possible. Based on this evidence, adjuvant chemotherapy has been hypothesized
to have a similar effect in children with spinal cord low-grade astrocytomas as well. In this study,
we have reviewed the patients with low-grade brain and spinal cord
astrocytoma to compare the response rate to therapy.
Table 1
Table 1
Treatment and outcome summary of all patients with low-grade astrocytoma of the brain and spinal cord.
Materials and Methods
A retrospective chart review was performed of all patients
diagnosed and treated for low-grade central nervous system
astrocytoma from 2000 to 2013 at our institution. Any patient
diagnosed with a grade I or II astrocytoma was included. Patients were
classified as brain astrocytoma if they had a tumor of the cerebrum,
cerebellum or brainstem. Patients were classified as spinal cord
astrocytoma if the tumor was in the spinal cord. Data were collected
from radiographic, pathological, operative and clinic reports. These
were used to determine demographics, histological diagnosis, extent
of resection, treatment received, and duration of follow-up and
relapse and progression status. The Children’s Hospital of Eastern
Ontario Research Ethics Board approved this study.
Response to therapy was determined by evidence of an objective
decrease in tumor size or a lesion becoming non-enhancing. Time
to progression was defined as time from diagnosis to evidence of
tumor growth or recurrence on magnetic resonance imaging (MRI).
Following completion of therapy (either surgery, chemotherapy or
radiation therapy), patients underwent routine follow up neurologic
examination and imaging every 3 months for the first 24 months
off therapy then every 6 months for the subsequent 24 months then
yearly.
Results
A total of 48 patients were found to have a histological
diagnosis of LGA, 5 with LGA of the spinal cord and 43 with LGA
of the brain. Forty-two (87.5%) patients were diagnosed with WHO
Grade I astrocytoma (41 pilocytic astrocytoma, 1 desmoplastic
astrocytoma), 10% with WHO Grade II (1 fibrillary astrocytoma, 2
oligoastrocytoma, 2 pilomyxoid astrocytoma), and 1 patient with an
unspecified low-grade astrocytoma. Of the patients with LGA of the
spinal cord, there were 2 with pilocytic astrocytoma, and 1 each with
fibrillary astrocytoma, oligoastrocytoma and unspecified low-grade
astrocytoma. Two patients had metastatic disease: 1 with metastasis
from the spinal cord to the brain, and the other, with metastasis
from the brain to the spinal cord. The median age at diagnosis for all
patients was 8.2 years (3.9 and 8.5 years for spine and brain patients,
respectively), 52.1% being female.
Patients with LGA of the brain presented most commonly with
headache (57.8%), vomiting (55.8%), gait disturbance (32.6%),
head tilt and/or vision changes (30.2%) and hemiparesis (11.6%).
Patients with LGA of the spinal cord presented with bowel or bladder
dysfunction (60%), gait disturbance (40%), hemiparesis (40%) and
foot drop or eversion (40%).
All patients had MRIs of the brain and spinal cord. The majority
of patients (83.3%) had precise initial tumor measurements on MRI,
and 46.5% of patients had precise tumor measurements after initial
treatment. The tumor was located in the cervical spinal cord in 1
patient, the thoracic spinal cord in 2 patients, and both cervical and
thoracic spinal cord in 2 patients. Of those with LGA of the brain,
the tumor was supratentorial in 15 (34.8%) patients, infratentorial in
26 (60.5%) patients, and both infra- and supratentorial in 2 (4.7%)
patients.
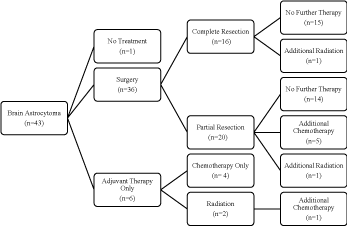
The treatment and outcome summary of all patients is shown
in Table 1. Thirty-nine (81.3%) patients underwent surgical
resection and 16.7% underwent biopsy only. Of all patients who
underwent surgical resection, 89.7% (n=35) were diagnosed with
pilocytic astrocytoma. One spinal cord patient and 16 brain patients
underwent complete resection, and 2 and 20, respectively, underwent
partial resection. Fourteen patients (82.4%) who underwent complete
resection had a histological diagnosis of pilocytic astrocytoma, while
the remaining were diagnosed with pilomyxoid astrocytoma (11.8%)
or fibrillary astrocytoma (5.9%). Twenty-one patients who underwent
partial resection were diagnosed with pilocytic astrocytoma (95.5%),
and 1 with oligoastrocytoma. Of the 22 patients with a partial tumor
resection (56.4%), 31.8% and 4.5 % received adjuvant chemotherapy
and radiotherapy, respectively. Of all patients, five (13.2%) received
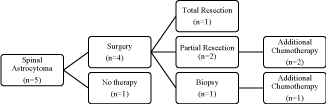
chemotherapy, without surgical resection (Figure 1 and 2). Fourteen
(82.4%) of those with complete resection remained progression free:
12 with pilocytic astrocytoma, 1 with fibrillary astrocytoma and 1
with pilomyxoid astrocytoma.
Of the 5 spinal cord tumor patients, 2 with partial resection and
1 with biopsy only received chemotherapy, and all responded to
therapy. Tumor size decreased by a mean of 36.9%, and no patients
relapsed. All 3 received carboplatin/vincristine regimens. The 2
patients not treated with chemotherapy had initially undergone
complete resection or biopsy with ventriculo-peritoneal shunt
placement. They showed tumor progression at a mean of 29.5
months and subsequent chemotherapy resulted in either a decrease
in tumor size or no evidence of remaining tumor at 7.5 years followup
(mean). No spinal cord tumor patients received radiation therapy.
Ten (23.3%) brain tumor patients received chemotherapy, 6 of
which were adjuvant treatment following partial resection (n=5) or
radiation (n=1). Of these 6, 4 responded to treatment, 1 progressed,
and 1 was transferred out of our institution. Seventy-five percent
of those who only received chemotherapy responded to treatment.
The chemotherapy regimens included carboplatin/vincristine (n=5),
vinblastine (n=2), vinblastine/vinorelbine (n=1), temozolamide
(n=1) and procarbazine/thioguanine/lomustine/vincristine (n=1).
Four (9.3%) brain tumor patients received radiation, either following
complete (n=1) or partial resection (n=1), prior to chemotherapy
(n=1, mentioned above) or as sole treatment (n=1). The patient
who underwent complete resection prior to radiation relapsed twice
and died. The remaining patients responded to therapy and did not
relapse at a mean of 5.5 years of follow-up (range 2.6-8.8 years). All
4patients received radiation at a dose of 54 Gray in 30 fractions. Of
all brain tumor patients, tumor size decreased by a mean of 44.5% for
those with surgery and adjuvant therapy, and 27.1% for those with
adjuvant therapy alone.
Ten (23.3%) brain tumor patients relapsed at a mean of 9.6
months after diagnosis. Those treated underwent resection (n=3) or
adjuvant therapy (n=5). Three (60%) of the latter and one (33.3%) of
the former relapsed again. The one patient was treated with radiation,
and remained progression-free at last follow-up. Of the 3 patients who
relapsed after adjuvant therapy, 1 died, 1 was transferred to another
institution, and 1 remained on chemotherapy at last follow-up.
There were 5 patients with WHO Grade II tumors: 1 fibrillary
astrocytoma, 2 oligoastrocytoma, and 2 pilomyxoid astrocytoma.
Three underwent a complete resection, of which one relapsed and 2
had a good outcome. One underwent partial resection and was treated
with chemotherapy with good outcome, and 1 underwent biopsy with
shunt, relapsed due to alternative therapy initially but then received
chemotherapy and had a good outcome.
There were 17 (36.9%) patients who continued to have symptoms
3 months after initial therapy was completed. Of these, 58.8% had
undergone partial resection with or without adjuvant treatment,
29.4% had undergone complete resection, and 11.8% had received
only adjuvant treatment. The most common symptoms were
headaches (41.2%), dysmetria (29.4%), hemiparesis (17.6%) and
ataxia (9.3%). Twelve (26.1%) of these patients developed symptoms
not present at diagnosis. Patients were followed for a mean of 5.4
years (range 3 months - 10.9 years).
Figure 1
Figure 2
Discussion
The management of low-grade spinal cord astrocytomas remains
challenging. Surgical resection can be difficult due to the infiltrative
nature of these tumors, and may result in long-term morbidity. While
chemotherapy and radiation have been shown to be of benefit in LGA
of the brain, their benefit in LGA of the spinal cord remains unclear.
We have shown in this small study that chemotherapy may be of
better benefit to patients with LGA of the spinal cord compared to
those with LGA of the brain, as all 5 patients with spinal cord LGA
responded to chemotherapy.
WHO Grade I and II pathologic classification of patients in this
series found that 5 of 48 had Grade II and the remainder Grade I.
The one patient who died had a Grade II astrocytoma in the brain.
Of the relapsed patients, 2 were Grade II and 8 were Grade I, thus
20% of Grade II and 19% of Grade I patients relapsed, demonstrating
no large difference among the two groups in terms of relapse rate,
although numbers are too small to measure significance.
When feasible, gross total resection may be curative without
adjuvant treatment [5]. Several studies have associated complete
resection with decreased rates of tumor progression and survival
rates of 90% or greater [11-14]. Among these, Fisher et al. [12],
found that all children with low-grade gliomas who underwent gross
total resection and 58% of those with a partial resection remained
progression-free at a mean follow-up of 7.3 years [12]. In our study,
of the 15 patients who only underwent gross total resection, 86.7%
remained progression free at a mean follow-up of 5.6 years. Studies
have reported an increased risk of neurological injury following
total resection [15,16], and as such, it has been argued that resection
should not sacrifice function [17,18]. We observed that the majority
of patients who experienced symptoms post-operatively were those
who underwent partial resections with or without adjuvant treatment
(58.8%). By contrast, 29.4% of patients with complete resections
experienced symptoms following surgery.
While some studies have found that adjuvant radiotherapy is
of benefit to pediatric low-grade spinal cord astrocytomas [19-21],
its effectiveness remains uncertain due to the lack of randomized
trials. Possible adverse effects from radiotherapy include spinal
deformities, myelitis, decreased fertility and an increased risk of
second malignancy [6]. As such, it is often not considered as primary
adjuvant therapy in children [6]. No patients in our study with LGA
of the spine received radiation therapy, and all had good outcomes,
demonstrating that it can be avoided in this tumor.
Chemotherapy has increasingly replaced radiotherapy as firstline
adjuvant treatment for LGA [10]. Thirteen patients received
chemotherapy, 3 of which had low-grade astrocytoma of the spinal
cord. The latter are of interest as no large or randomized trials have
documented its effect on low-grade spinal cord astrocytomas. However,
several small-scale reviews have recorded a response to chemotherapy
in pediatric patients with these tumors [3,4,6, 24-27]. Eight patients,
including the 3 with spinal cord astrocytoma, received a carboplatin
and vincristine regimen. All responded to treatment. One patient
with astrocytoma of the spinal cord developed an allergic reaction
to carboplatin, and was substituted with actinomycin D. Carboplatin
and vincristine have been shown to be effective in treating low-grade
astrocytomas of the brain [22,23]. The Children’s Oncology Group
performed a randomized trial comparing the response to regimens
of vincristine/carboplatin and thioguanine/procarbazine/lomustine/
vincristine in young children with low-grade gliomas of the brain,
and found these regimens to have similar 5-year event-free survival
[23]. Regimens consisting of carboplatin and vincristine [4,27], or
carboplatin alone [6,25], have been reported to be of benefit in other
studies of pediatric patients with low-grade spinal cord astrocytoma.
Lowis et al. [27], found a good response to carboplatin and vincristine
in a 4-year-old child with spinal astrocytoma without radiation.
Townsend et al. [4], also presented 4 spinal tumor patients treated
with carboplatin and vincristine, after which disease stabilization or
minor response was observed. Chamoun et al. [3], reported on two
patients with progressive low-grade spinal cord astrocytomas initially
treated with surgery and radiation. Both showed tumor stabilization
following treatment with temozolamide.
Overall, our institutional experience shows that the use of
chemotherapy in pediatric patients with low-grade astrocytomas
in the spinal cord is of benefit to newly diagnosed and progressing
tumours. As with other studies, our population is small and largescale,
national studies are warranted.
Conclusion
This study reviewed 48 pediatric patients diagnosed with low-grade astrocytomas of the brain and of the spinal cord, and compared their response to therapy. We found that all patients with low-grade astrocytomas of the spinal cord had a response to adjuvant chemotherapy and none received radiation therapy, while only 81.8% of those with astrocytomas of the brain had a response to adjuvant therapy including radiation therapy in 9%. Due to the rarity of these tumors occurring in the spinal cord, larger scale studies are necessary to confirm the effectiveness of adjuvant therapy.
References
- Rodriguez FJ, Lim KS, Bowers D, Eberhart CG. Pathological and molecular advances in pediatric low grade astrocytoma. Annu Rev Pathol. 2013; 8: 361-379.
- Scheinemann K, Hukin J. Low Grade Glioma. In: Scheinemann K, Bouffet E. Pediatric Neuro-oncology. Springer. 2015; 91-99.
- Chamoun RB, Alaraj AM, Al Kutoubi AO, About MR, Haddad GF. Role of temozolamide in spinal cord low grade astrocytomas: results in two paediatric patients. ActaNeurochir (Wien). 2006; 148: 175-179.
- Townsend N, Handler M, Fleitz J, Foreman N. Intramedullary spinal cord astrocytomas in children. Pediatr Blood Cancer. 2004; 43: 629-632.
- Sievert AJ, Fisher MJ. Pediatric low grade gliomas. J Child Neurol. 2009; 24: 1397-1408.
- Hassal TE, Mitchell AE, Ashley DM. Carboplatin chemotherapy for progressive intramedullary spinal cord low-grade gliomas in children: three case studies and a review of the literature. Neuro Oncol. 2001; 3: 251-257.
- Epstein F, Epstein N. Surgical treatment of spinal cord astrocytomas of childhood: a series of 19 patients. J Neurosurg. 1982; 57: 685-689.
- Goh KY, Velasquez L, Epstein FJ. Pediatric intramedullary spinal cord tumors: is surgery alone enough? PediatrNeurosurg. 1997; 27: 34-39.
- Wisoff JH, Sanford RA, Heler LA, Sposto R, Burger PC, Yates AJ, et al. Primary neurosurgery for pediatric low-grade gliomas: a prospective multiinstitutional study from the Children’s Oncology Group. Neurosurgery. 2011; 68: 1548-1555.
- Scheinemann K, Bartels U, Huang A, Hawkins C, Kulkarni AV, Bourret E, et al. Survival and functional outcome of childhood spinal cord low-grade gliomas. J Neurosurg Pediatrics. 2009; 4: 254-261.
- Pollack IF, Claassen D, al-Shboul Q, Janosky JE, Deutsch M. Low-grade gliomas of the cerebral hemispheres in children: an analysis of 71 cases. J Neurosurg. 1995; 82: 536-547.
- Fisher BJ, Leighton CC, Vujovic O, Macdonald DR, Stitt L. Results of a policy of surveillance alone after surgical management of pediatric low grade gliomas. Int J RadiatOncolBiol Phys. 2001; 51: 704-710.
- Gajjar A, Sanford RA, Heideman R,Langston JA, Sanford RA, Walter A, et al. Low-grade astrocytoma: a decade of experience at St. Jude Children’s Research Hospital. J ClinOncol. 1997; 15: 2792-2799.
- Fisher PG, Tihan T, Goldthwaite PT, Wharam MD, Carson BS, Weingart JD, et al. Outcome analysis of childhood low-grade astrocytomas. Pediatr Blood Cancer. 2008; 51: 245-250.
- Babu R, Karikari IO, Owens TR, Bagley CA. Spinal cord astrocytoma: a modern 20-year experience at a single institution. Spine. 2014; 39: 533-540.
- Epstein FJ, Farmer JP, Freed D. Adult intramedullary astrocytomas of the spinal cord. J Neurosurg. 1992; 77: 355-359.
- Lee HK, Chang EL, Fuller GN, Aldape KD, Atkinson GJ, Levy LB, et al. The prognostic value of neurologic function in astrocytic spinal cord glioma. Neuro Oncol. 2003; 5: 208-213.
- Innocenzi G, Salvati M, Cervoni L, Delfini R, Cantore G. Prognostic factors in intramedullary astrocytomas. ClinNeurolNeurosurg. 1997; 99: 1-5.
- Huddart R, Traish D, Ashley S, Moore A, Brada M. Management of spinal astrocytomas with conservative surgery and radiotherapy. Br J Neurosurg. 1993; 7: 473-479.
- O’Sullivan C, Jenkin RD, Doherty MA, Hoffman HJ, Greenberg ML. Spinal cord tumours in children: long-term results of combined surgical and radiation treatment. J Neurosurg. 1994; 81: 507-512.
- Minehan KJ, Shaw EG, Scheithauer BW, Davis DL, Onofrio BM. Spinal cord astrocytoma: pathological and treatment considerations. J Neurosurg. 1995; 83: 590-595.
- Packer RJ, Ater J, Allen J,Phillips P, Geyer R, Nicholson HS, et al.Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. 1997; 86: 747-754.
- Ater JL, Zhou T, Holmes E, Mazewski CM, Booth TN, Freyer DR, et al. Randomized study of two chemotherapy regimens for treatment of lowgrade glioma in young children: a report from the children’s oncology group. J ClinOncol. 2012; 30: 2641-2647.
- Doireau V, Grill J, Zerah M, Lellouch-Tubiana A, Couanet D, Chastagner P, et al. Chemotherapy for unresectable and recurrent intramedullary glial tumours in children. Br J Cancer. 1999; 81: 835-840.
- Merchant TE, Kiehna EN, Thompson SJ, Heideman R, Sanford RA, Kun LE. Pediatric low-grade and ependymal spinal cord tumours. Pediatr Neurosurg. 2000; 32: 30-36.
- Mora J, Cruz O, Gala S, Navarro R. Successful treatment of childhood intramedullary spinal cord astrocytomas with irinotecan and cisplatin. Neuro Oncol. 2007; 9: 39-46.
- Lowis SP, Pizer BL, Coakham H, Nelson RJ, Bouffet E. Chemotherapy for spinal cord astrocytoma: can natural history be modified? Childs Nerv Syst. 1998; 14: 317-321.