Research Article
A Quantitative Assessment of Imaging Frequency on the Treatment Setup Accuracy in TomoTherapy
Bichay TJ1,2*, Davis S1, Mayville AH1 and Bichay NDT3
1Mercy Health Saint Mary’s, The Lacks Cancer Center, Grand Rapids Michigan, USA
2Wayne State University School of Medicine, USA
3Department of Political Science, Michigan State University, USA
*Corresponding author: Tewfik J. Bichay, Department of Radiation Oncology, The Lacks Cancer Center, Mercy Health, Saint Mary’s, 200 Jefferson S.E., Grand Rapids, Michigan, 49503, USA
Published: 31 Aug, 2016
Cite this article as: Bichay TJ, Davis S, Mayville AH, Bichay
NDT. A Quantitative Assessment of
Imaging Frequency on the Treatment
Setup Accuracy in TomoTherapy. Clin
Oncol. 2016; 1: 1064.
Abstract
Image guided radiation therapy (IGRT) is becoming the standard of practice for many treatment sites and techniques, especially those involving high dose gradients such as stereotactic radiosurgery (SRS), stereotactic radiotherapy (SBRT) and intensity modulated radiotherapy (IMRT). The purpose of this study is to quantify the setup accuracy for various IGRT frequency protocols from tattoo-only setups with no imaging, to imaging every fifth, fourth, third, second fraction, as well as daily imaging prior to TomoTherapy IMRT treatment. Total vector shifts were calculated from the lateral, longitudinal and vertical (x,y,z) displacements and the mean shift error for the various protocols analyzed for five treatment sites: cranial, head and neck, prostate, prostate bed and lung. On a given non-imaging day the shift relative to tattoos was determined by using the most recent imaged shift values and applying these to the current setup. Imaging data from 260 patients was analyzed for a total of 8,379 treatment sessions with displacement in the lateral, longitudinal and vertical directions. Lung patients and prostate patients had the largest vector shifts with a mean daily displacement of 10.4 mm. Prostate bed patients had an average vector shift of 9.0 mm, while head and neck and cranial patients had an average shift of 6.9 mm and 5.6 mm respectively. Increasing the imaging frequency increased the accuracy of the setup. Even if imaged every second day there is still an average error of 3.8 mm in the setup of cranial patients and 11.5 mm for lung patients ten percent of the time. Our data demonstrates that for TomoTherapy treatments, daily imaging is advisable for the five treatment sites presented in this study.
Introduction
One of the more common goals of modern radiation therapy has been to more accurately
deliver dose to a target while minimizing unnecessary radiation dose to surrounding normal
tissues [1]. Gaining the ability to visually locate the region of interest, and precisely align the
radiation delivery to the target has greatly improved the accuracy and efficacy of treatments [2].
Image guided radiation therapy (IGRT) has become a common tool for daily patient alignment.
Modern radiotherapy machines have adopted the ability to quickly acquire planar x-ray images for
comparison to planning digitally reconstructed radiographs (DRR) and/or 3D volumetric images
(cone beam or helical CT) for reference to the original CT [2].
Historically, aligning a patient for radiation therapy consisted of matching fixed in-room lasers
to specific locations on the patient, often identified by tattoos placed during simulation. In order to
accommodate daily variations in setup alignment according to tattoos; significant setup margin is
added to the target contour to prevent missing the target during treatment delivery [3]. The sacrifice
of additional dose to surrounding normal tissues is necessary in order to ensure adequate delivery
of target dose [3]. In 3D therapy typical penumbra about a target is approximately 8-15 mm. In
IMRT, however, the falloff may be only a few millimeters [4]. Small positional errors that were of
little consequence in 3D planning may now have serious consequences in IMRT [5]. In head and
neck treatments for example it is typical to have a 70 Gy target within a few millimeters of a much
more sensitive parotid gland or spinal cord.
IGRT has dramatically changed the way patients are setup on a daily basis, and therefore
changed the inherent accuracy of treatment delivery. The ability to fine-tune the initial tattoo
setup, according to the exact location of the target and nearby normal tissues, reduces the error
in daily dose delivery. The intent of this study is to evaluate this gain in accuracy of IGRT using
megavoltage helical CT scans (MVCT) for TomoTherapy procedures over simple tattoo setups. The
daily three-dimensional shifts necessary to bring a patient’s anatomy
into alignment, following initial tattoo setup, have been collected
for several treatment sites including lung, prostate, prostate bed
following prostatectomy, head and neck, and cranium. An analysis
of several imaging frequency protocols summarizes the increase in
treatment accuracy provided by IGRT over traditional alignment to
tattoos only.
Materials and Methods
All Patients were set up on a TomoTherapy HiArt (Accuray,
Sunnyvale CA) treatment couch and aligned to lasers using skin
tattoos, or marks applied to the patient's mask at time of simulation.
Prior to treatment, patients were imaged with a helical megavoltage
CT (MVCT) with a pixel resolution of 512 x 512. The imaging
system gives an initial automatic prediction of shifts necessary to
align patients to the treatment plan reference conditions based on
the protocol chosen by the therapists. Therapists choose between
bony anatomy only, bony and soft tissue anatomy, or the full image
technique, which compares high and low densities, as well as air data
points over the entire image for alignment. Following automatic
alignment, additional manual shifts may then be applied to fine tune
the treatment position based on the target region of interest or the
presence of fiducial markers.
Rotations were accounted for and corrected in all patients,
however for this study only translational offsets were statistically
analyzed. Rotations about the x and z axes, pitch and yaw respectively,
were corrected for manually by the therapists through physical
movements of the patient if necessary. TomoTherapy has the unique
ability to correct for rotations about the y axis, or roll, by varying the
starting gantry angle position [6]. Because the TomoTherapy system
has only the ability to automatically correct for roll rotations, the
registration procedure gives the operator the choice of computing
translations only, translations with roll only, translations with yaw
only, translations plus roll and yaw, or translations plus roll, yaw,
and pitch. In all cases we used a protocol of translation with roll
only applied to bony anatomy or bony and soft tissue. In the case of
lung patients where the tumor could be readily seen the therapists
shifted the patient to ensure best alignment was in the tumor region.
Similarly, for prostate patients the setup position was optimized to
place the fiducials at the reference position.
At first release TomoTherapy offered fine, normal, and coarse
imaging protocols, of slice thickness 2 mm, 4 mm, and 6 mm
respectively. We developed, along with TomoTherapy, an "ultrafine"
protocol of 1 mm slice thickness specifically for intracranial SRS
treatments [7,8]. For all cranial cases the imaging protocol chosen
was ultra-fine. For all other treatment sites the imaging protocol was
typically fine, though normal or coarse may be chosen for patients
with very large tumors and therefore long scan regions. Scan length
was determined by the therapists in order to adequately visualize
the treatment region and any anatomical landmarks critical for
alignment.
The kilovoltage CT (KVCT) images to which daily MVCT datasets
were compared were acquired on a Siemens Sensation Open 40-slice
wide bore CT scanner (Siemens Medical Solutions, Malvern, PA). For
planning purposes, the KVCT, acquired at 512 x 512 resolution, was
resampled to 256 x 256, which is also the image set used for daily
alignment.
Boswell et al investigated the accuracy of the TomoTherapy
automatic registration procedure. They determined the accuracy,
excluding outliers, to be within one half of the CT voxel size in
all translational directions. They also determined that manual
registration was at best as accurate as automatic registration, but
typically inferior. The main benefit of manual evaluation is to detect
outliers and fine-tune the automatic registration based on specific
landmarks.
Treatment sites
There were five treatment sites studied. Table 1 summarizes
the number of patients and the total number of treatments in each
category.
Cranial and head and neck patients were setup on a customformed
pillow fixed onto an S-frame support (Civco, Coralville,
IA), and with their arms to their sides. A custom-molded reinforced
aquaplast (Civco) mask ensured minimal patient movement during
the treatment procedure. Registration was accomplished utilizing
agreement in bony anatomy.
Because of the difficulty in visualizing the prostate under MVCT,
prostate patients were implanted with three visicoil gold markers
(IBA Dosimetry, Bartlett, TN) prior to simulation. Following initial
automatic alignment to bony anatomy, the fiducials aid the therapists
in determining accurate prostate position during treatment. Both
prostate and prostate bed patients were positioned with their legs
placed in a Vac Loc custom-molded immobilizer (Civco). Prostate
bed patients were aligned based on bony anatomy.
Lung patients were setup on a combination of Vac Loc support
under the thorax with arms up in a Wing board (Civco). Lung patient
alignment was based on bony anatomy and soft tissue with manual
emphasis on agreement of the tumor region.
Shift calculation
The final treatment position is reported as a net movement from
the initial lateral, longitudinal and vertical position (x,y,z). Shifts in
x,y and z directions were analyzed at each treatment fraction for a
total of 8,379 treatments. For each imaging session the resultant
vector shift (v) was calculated from the analysis of:
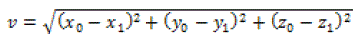
Imaging protocols
The resultant mean setup error for various imaging protocols
was calculated using Stata analysis software (Stata/Mp 14, Statacorp
College Station, TX). For all treatments all patients were imaged
daily and shifted to the appropriate position prior to treatment. We
simulated the error in setup that would result if various imaging
frequency protocols were used. For example, to simulate the setup
error if imaging was carried out once every five days, we used the
shift position carried out on the reference day for the four subsequent
non-imaging days. The resultant error is the difference between the
imaging day position and the actual position on the simulated nonimaging
day. The following protocols were studied:
Daily imaging
Patients were imaged every day. The mean error in setup position
is assumed to be zero since any offsets determined by imaging were
applied prior to treatment.
Every second day
Patients imaged every second day. Patient initially set up based
on tattoos and the reference position determined on the previous
imaging day was applied. The difference between this position, and
the actual shift determined at time of imaging on this day is the
resultant error.
Every third day
Patients imaged on one day and this offset value was used for the
next two days.
Every fourth day
Patients imaged on one day and this offset value was used for the
next three days.
Every fifth day
Patients imaged every fifth fraction and this offset value was used
for the next four days.
No imaging
If no imaging was ever carried out then the positional error would
be equal to the calculated displacement for each treatment day.
Table 1
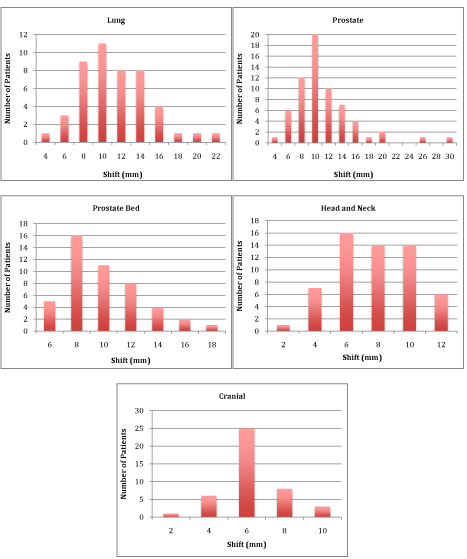
Figure 1
Figure 1
Frequency histogram of average per patient vector shifts. The values represent the mean vector position error for
each patient for the total population of patients for each treatment site.
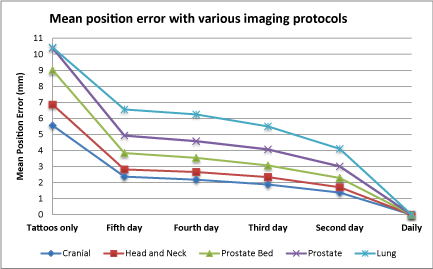
Figure 2
Figure 2
Mean position setup error with varying imaging frequency for
the five treatment sites studied. The mean position error is the difference
between the vector shift required based on IGRT and the tattoo position.
Results
The daily MVCT is used to determine the extent of patient
movement from the initial tattoo-laser based setup to an alignment
matching patient anatomy in the treatment plan. If one assumes
worst-case scenario where no imaging is done, the mean vector error
over a course of treatment would be calculated from the shift error of
each day. This data is presented in the series of histograms in Figure
1 for average patient shift required to correct for the setup error.
The average displacement error for the total number of treatments
for all patients for each imaging protocol is summarized in Table
2. The mean displacement error is least for cranial cases (5.6 mm)
since these patients are fixed in a mask immobilizer which results in
minimal movement relative to external marks. Similarly head and
neck patients are also immobilized in a mask but show somewhat
larger movements (6.9 mm). The greatest mean correction is that
of prostate (10.4 mm) and lung (10.4 mm) patients where internal
anatomy relative to external marks would be expected to be least stable
of the sites studied. Prostate bed patients are setup day-to-day based
on bony landmarks, which may be expected to have less variation (9.0
mm) compared to the soft tissue of prostate patients [9].
If imaging is carried out once per week, and the measured
displacement is applied on the imaging day as well as to the next
four fractions, there is improvement in setup accuracy compared to
no imaging at all. For example the mean setup error in the prostate
population decreases from 10.4 mm (Table 2) if never imaged to 4.9
mm for imaging once a week. Imaging every fourth day results in a
further drop to 4.6 mm, then to 4.1 mm when imaged every third
day and if imaged every second day the mean error drops to 3.0 mm.
For the purpose of this study applying the shifts determined at daily
imaging is assumed to result in no residual setup error. The data in
Table 2 demonstrates a similar pattern for the other four sites studied
in this paper. The prostate bed results demonstrate a reduction in
setup error from 9.0 mm if never imaged to 3.8 mm if imaged once
a week. Imaging every fourth day reduces the error to 3.5 mm, and
every third day further reduces the error to 3.1 mm, and down to 2.3
mm if imaged every second day. The cranial results have a similar
pattern but less magnitude where imaging once a week reduces the
mean error from 5.6 mm to 2.4 mm, and for once every four days the
error drops to 2.1. Every third day results in a further drop to 1.9 mm
and every second day reduces it further to 1.4 mm. The head and neck
setup error was 6.9 mm if no imaging is carried out then drops to
2.8 mm for every fifth day, 2.7 mm for every fourth day 2.3 for every
third day and 1.7 mm for every second day. Lung patient’s setup error
dropped from 10.4 mm with no imaging, to 6.6 mm with every fifth
day imaging, to 6.2 mm with imaging every fourth day, 5.5 mm if
imaged every third day, and 4.1 mm if imaging was carried out every
second day. A graphical summary of the improvement in setup error
is presented in Figure 2. The residual mean setup error for the various
imaging protocols is demonstrated when moving from setup only on
tattoos, to protocols involving various imaging frequencies.
The calculation of mean errors is useful in understanding the
average magnitude of error involved in the various protocols. We
were also interested in determining the amount of deviation that
would occur in the worst case 10% of the treatment sessions for each
one of these protocols. The data is presented in Table 2. For prostate
patients if no imaging were carried out at all then ten percent of the
time the mean error in displacement would be 16.8 mm. If imaging
is carried out once per week then this error would drop to 9.9 mm
then to 9.5 mm for imaging every fourth day or every third day and
if imaged every second day it would be reduced to 8.2 mm. In the
case of cranial patients if imaged every second day there would still
be an average of 3.8 mm error in ten percent of the treatments. Lung
patients had the greatest mean error where imaging once a week
would result in a mean error of 14.6 mm ten percent of the time, and
imaging every second day would still have an error of 11.5 mm ten
percent of the treatments.
Table 2
Table 2
Average error induced by varying the frequency of setup imaging, and
the value which 10% of patients would experience errors in excess of.
Discussion and Conclusion
The smallest mean patient shift was for cranial and head and
neck patients. The result is expected as both anatomical regions are
immobilized by a thermoplastic mask and relied largely on bony
anatomy. The shift for head and neck is somewhat larger than cranial
(6.9 mm vs. 5.6 mm) which is likely due to a longer region being
treated. The longer region includes a flexible cervical spine, which
can lead to some setup variation and may result in a registration
bias from one therapist to another. The greatest shifts were for lung
and prostate. In the case of prostate the shift was done to place the
implanted fiducial markers back to the reference position at time of
planning. Similarly in the case of lung the shift was to align the tumor
with the reference position of the plan. Shifts were intermediate for
prostatic bed where the alignment was based on bony anatomy as
opposed to soft tissue.
Our results demonstrate that the error associated with various
imaging protocols is seen to decrease with increased frequency of
imaging. These results are similar to those of Kupelian et al. [10] who
studied different imaging protocols for prostate patients. Treatment
margins for IMRT will vary by treatment site and treatment modality
being used. In the case of TomoTherapy for example with IGRT
our center uses 3 mm setup uncertainty in all directions for cranial
treatments. Even for frequent imaging of every second day there will
still be a mean error greater than our margin in ten percent of the
treatments. This effectively means that a geometric miss would occur
in more than one in ten treatments. Similar results were found for
the other four anatomical sites observed. We use a 5 mm margin for
prostate except for the posterior aspect where 3 mm is used. Our data
demonstrates that any imaging frequency other than daily would
result in an error of greater that 8 mm in ten percent of treatments.
Similarly a margin of 5-10mm PTV may be applied to lung protocols
using IMRT. Our data demonstrates that for these lung patients the
setup error would be 11.5 mm even if imaging was done every other
day. This would suggest that with TomoTherapy, and perhaps other
treatment systems where very conformal distributions are used,
daily imaging is essential for acceptable target coverage without the
addition of excessive margins. We have shown previously the error
associated with setup on tattoos only for cranial [11] and for lung
patients [12]. Previous publications have shown that setup position
errors have both a random and a systematic component [13]. Our
data agrees with this conclusion as the daily setup variation appears
to have a random component since using a correction based on a
previous day does not fully correct for the setup uncertainty. Schubert
et al. [14] have taken an approach whereby the displacements for
prostate patients over the first four days are used to generate a custom
PTV. This approach appears effective at minimizing geometric miss
with less frequent imaging; however it is at the cost of modifying
each patient’s PTV to account for the setup uncertainty. The present
study limited the corrections in setup to translation and roll rotation
only. Other rotation corrections for yaw and pitch could not be easily
applied as the TomoTherapy couch is not readily amenable to allow
this movement. This is discussed by Schubert el al. [13] who suggests
that table sag would likely result in pitch variation and yaw may result
in lateral displacement corrections.
The use of IGRT has allowed treatment margin reductions,
which leads to a decrease in the volume of normal tissue treated.
This reduction in normal tissue volume results in a decrease in the
complication rate [15]. The use of IGRT ensures that this reduction in
the complication rate is not at the cost of decreased tumor response.
Several authors have demonstrated that with improved imaging both
reductions in complication rates and increased tumor response can
coexist [15]. Our data compares the improvement in setup accuracy
with the frequency of imaging the patient prior to treatment.
Intuitively one would predict that increased imaging frequency would
result in increased treatment accuracy. Our data confirms this and
assigns a numeric value to this increase in setup accuracy. The data
for the five treatment sites studied is in agreement with a previous
publication studying imaging frequency for prostate patients [10].
Given the results of this study daily imaging for TomoTherapy is
advised for acceptable treatment accuracy for the five treatment sites
studied.
References
- Levitt SH, Purdy JA, Perez CA, Poortmans P. Technical Basis of Radiation Therapy: Practical Clinical Applications, 4th edition. Springer. 2006. 167.
- Wu VW, Law MY, Star-Lack J, Cheung FW, Ling CC. Technologies of image guidance and the development of advanced linear accelerator systems for radiotherapy. Front Radiat Ther Oncol. 2011; 43: 132-164.
- Tanyi JA, Tongming H, Summers PA, Mburu RG, Kato CM, Rhodes SM, et al. Assessment of planning target volume margins for intensity-modulated radiotherapy of the prostate gland: role of daily inter- and intrafractionation motion. Int J Radiat Oncol Biol Phys. 2010; 78: 1579-1585.
- val Vulpen M, Field C, Raaijmakers CP, Parliament MB, Terhaard CH, MacKenzie MA, et al. Comparing step-and-shoot IMRT with dynamic helical tomotherapy IMRT plans for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2005; 62: 1535-1539.
- Hong TS, Tomé WA, Chappell RJ, Chinnaiyan P, Mehta MP, Harari PM. The impact of daily setup variations on head-and-neck intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2005; 61: 779-788.
- Boswell S, Tomé W, Jeraj R, Jaradat H, Mackie TR. Automatic registration of megavoltage to kilovoltage CT images in helical tomotherapy: an evaluation of the setup verification process for the special case of a rigid head phantom. Med phys. 2006; 33: 4395-4404.
- T Bichay, C Chen, J Meadows, D Schippers, D Lucas, K Ruchala, et al. Dosimetric and Image Quality Analysis of a New Ultrafine Imaging Mode in TomoTherapy. Medical Physics. 2008; 35: 2696.
- E Chao, T Bichay, D Lucas, K Ruchala, G Olivera. Longitudinal resolution of the TomoTherapy® MVCT image and potential improvements. Med Phys. 2008; 35: 2706.
- Ost P, De Meerleer G, De Gersem W, Impens A, De Neve W. Analysis of prostate bed motion using daily cone-beam computed tomography during postprostatectomy radiotherapy. Int J Radiat Oncol Biol Phys. 2011; 79: 188-194.
- Kupelian PA, Lee C, Langen KM, Zeidan OA, Mañon RR, Willoughby TR, et al. Evaluation of image-guidance strategies in the treatment of localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008; 70: 1151-1157.
- Bichay TJ, Ebrom P. A quantitative analysis of the reliability of aquaplast mask immobilization for cranial radiosurgery with TomoTherapy. World Congress on Medical Physics and Biomedical Engineering, IFMBE Proceedings. 2012; 39:1915-1918.
- Bichay T, Meadows J, Chen C, Klynstra N. A quantitative assessment of the improvement in lung treatment setup accuracy with IGRT in TomoTherapy. Med Phys. 2010; 37: 3152-3153.
- Schubert LK, Westerly DC, Tomé WA, Mehta MP, Soisson ET, Mackie TR, et al. A comprehensive assessment by tumor site of patient setup using daily MVCT imaging from more than 3,800 helical tomotherapy treatments. Int J Radiat Oncol Biol Phys. 2009; 73: 1260-1269.
- Beldjoudi G, Yartsev S, Bauman G, Battista J, Van Dyk J. Schedule for CT image guidance in treating prostate cancer with helical tomotherapy. Br J Radiol. 2010; 83: 241-251.
- Zelefsky MJ, Kollmeier M, Cox B, Fidaleo A, Sperling D, Pei X, et al. Improved clinical outcomes with high-dose image guided radiotherapy compared with non-IGRT for the treatment of clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2012; 84: 125-129.