Research Article
Ectopic Production of Β-hCG and Loss of P16 as a Predictor of Outcome in Patients with Newly Diagnosed Osteosarcoma
Nye L, Yeldandi A, Peabody T, Attar S, Salamon MA, Gandhi M, Gursel D, Rademaker A, Hayes JP and Agulnik M*
Division of Hematology/ Oncology, Robert H. Lurie Comprehensive Cancer Center Northwestern University
Feinberg School of Medicine, USA
*Corresponding author: Agulnik M, Division of Hematology/ Oncology, Robert H. Lurie Comprehensive Cancer Center Northwestern University Feinberg School of Medicine, 676 N. St. Clair Street, Suite 850 Chicago, USA
Published: 26 Aug, 2016
Cite this article as: Nye L, Yeldandi A, Peabody T, Attar S,
Salamon MA, Gandhi M, et al. Ectopic
Production of Β-hCG and Loss of P16
as a Predictor of Outcome in Patients
with Newly Diagnosed Osteosarcoma.
Clin Oncol. 2016; 1: 1061.
Abstract
Osteosarcoma is the most common malignant bone tumor in children and young adults and is
associated with high mortality. We investigated the expression of β- hCG and P16 in osteosarcoma
and correlated with outcomes.
Methods: Immunohistochemistry (IHC) for β-hCG was performed on diagnostic osteosarcoma
specimens and post-treatment specimens. IHC for P16 was performed on diagnostic specimens.
Results: Median age was 32. Median progression free survival (PFS) was 11.5 months. Median
overall survival (OS) was 38.0 months. Positive β-hCG staining on diagnostic specimens did not
correlate with percent tumor necrosis, 2 year PFS or OS. Patients with a post-treatment β-hCG
staining of ≥ 50% had a median PFS of 6.1 months vs 19.2 months in patients with β-hCG less than
50% (p = 0.03). Patients with a post-treatment β-hCG staining ≥ 50% also demonstrated a trend
toward shorter median OS (17.1 months vs 19.2). There was no statistically significant relationship
between P16 staining on diagnostic osteosarcoma specimens and post- treatment percent tumor
necrosis. Patients with negative P16 staining on diagnostic specimens had a lower 2 year PFS
compared to positive P16 staining (2 year PFS 0% vs 71%), p=0.022. There was a trend toward worse
2 year OS in patients with P16 negative diagnostic specimens compared to patients with P16 positive
tumors, 22% vs 86%.
Discussion: We have demonstrated feasibility and utility in examining P16 and β-hCG in
osteosarcoma. We found that post-treatment β-hCG expression correlated with poorer outcomes,
specifically worsened PFS. In congruence with previous reports, negative P16 staining confers worse
outcomes.
Introduction
Osteosarcoma while considered a rare cancer, is the most common malignant bone tumor in children and young adults [1] and comprises 28% of the bone cancers diagnosed in adults over the age of 40 [2]. Osteosarcoma is associated with a relatively high mortality rate and a 5 year overall survival of only 66.7% [1]. Several factors have been demonstrated to be prognostic including tumor location, size, patient age, metastatic disease, histological response to chemotherapy and surgical outcomes [3-5]. However there is little data on individualized tumor characteristics for predicting or monitoring response to chemotherapy. Ectopic production of β- hCG (human chorionic gonadotropin), by osteosarcomas is an uncommon phenomenon that has rarely been documented and few case reports have noted a trend towards poor outcomes [6-8]. Inactivation of P16 has also been associated with continuous cell proliferation in numerous malignancies and may correlate with worse outcomes in osteosarcoma [9,10]. We recently observed a case of a recurrent osteosarcoma associated with high levels of serum β-hCG, which normalized after tumor resection. Immunohistochemical staining for β-hCG established the tumor to be the source of the elevated serum maker. We have further investigated the expression of β-hCG and P16 in osteosarcoma tumors diagnosed at our institution and correlated these findings with clinical outcomes.
Materials and Methods
Thirteen adult patients with available pathologic specimens were diagnosed with osteosarcoma at our institution between 2006 and 2014. Retrospective chart review was conducted on included patients and demographic, clinical, pathological, radiological and laboratory data was collected. Previously stored formalin fixed and paraffin embedded blocks from available pre- treatment diagnostic specimens and the designated study pathologist reviewed posttreatment surgical specimens of included patients, and the most representative block was chosen for sectioning. The slides with the greatest proportion of tumor were then selected for staining. Two representative slides from the diagnostic specimen (one for β-hCG and one for P16 staining) and one representative slide from the post treatment resection specimen (only for β-hCG ) were selected per subject. Immunohistochemistry staining for β-hCG was performed on the formalin fixed, paraffin embedded tissue sections of the diagnostic osteosarcoma specimens and post treatment surgical resection specimens. Rabbit polyclonal anti-human β-hCG antibody (Dako, Carpinteria, California) was utilized with pH adjusted (pH 6.0) antigen retrieval. Placenta was used as positive control. Stained slides were examined by the study pathologist and analyzed for cytoplasmic staining intensity (absent or present) and frequency of expression (categorized as 0%, <10%, 10-50% or ≥ 50%) was reported. Both intensity and frequency of β-hCG expression was analyzed, as there is no established grading system for β-hCG staining pattern in osteosarcoma (Figure 1). Immunohistochemistry staining for P16 was performed on the formalin fixed, paraffin embedded tissue sections of the diagnostic osteosarcoma specimens. Mouse monoclonal anti-human P16INK4a antibody (Biocare Medical, LLC, Concord, California) was utilized with pH adjusted (pH 9.0) antigen retrieval. The positive control was cervical cancer specimens. Tonsil tissue microarrays were used for negative control. Both intensity and frequency of P16 expression was analyzed and reported. Specimens were classified as negative (absent) or positive (present) for P16 nuclear expression (Figure 1). Positive staining for P16 included specimens with ≥ 30% nuclear staining. This threshold was chosen based on prior reports of P16 staining in osteosarcoma [10,11]. Assessment of tumor necrosis is reported as a percentage and was obtained from post-treatment surgical specimen pathology report on chart review. Progression-free and overall survival was analyzed using Kaplan-Meier curves. Subgroups were compared using the log rank test. Comparisons of biomarkers between p16 positive and negative subgroups were done using the Wilcoxon rank sum test.
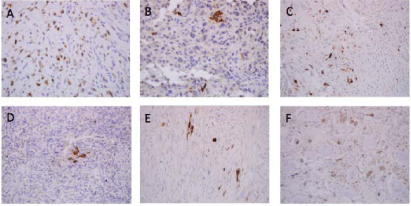
Figure 1
Figure 1
Representative images of immunohistochemistry and designated
categories of the analyzed biomarkers. A, P16 positive staining. B, Positive
β-hCG staining (<10% category) of diagnostic specimen. C, Positive β-hCG
staining (≥10- 50% category) of diagnostic specimen. D, Positive β-hCG
staining (<10% category) of post-treatment specimen. E, Positive β-hCG
staining (≥10- 50% category) of post-treatment specimen. F, Positive β-hCG
staining (≥50% category) of post-treatment specimen.
Results
Thirteen patients with available pathology were diagnosed with
osteosarcoma at our institution between 2006 and 2014. The median
age at diagnosis was 32 (range 20-64) including 7 females and 6
males. The median follow up time was 26.7 months (range 5.2 – 91.0).
Median progression free survival (PFS) was 11.5 months. Median
overall survival (OS) was 38.0 months. Further characteristics of
tumors described in (Table 1).
β-hCG
Ten patients had diagnostic specimens available for β-hCG
staining. Of these 10 patients included, 7 had post-treatment resection
specimens available with percent tumor necrosis detailed on final
pathology report. There was no statistically significant relationship
between β-hCG staining on the diagnostic osteosarcoma specimen
and percent tumor necrosis on resection specimen. Positive β-hCG
staining on the diagnostic specimen (n=6) did not correlate with
worse 2 year PFS (p=0.45) or OS (p=0.90). Eight patients had a posttreatment
tissue specimen available for β-hCG staining. Patients with
negative staining for β-hCG (n=4) had a 2 year PFS of 50% compared
with patients with positive β-hCG staining (n=4) whom had a 2
year PFS of only 25%. There was a trend toward worse 2 year PFS
and OS in patients with positive β- hCG staining on post-treatment
tissue, however the difference was not statistically significant. Given
there is no established grading system for β-hCG staining pattern in
osteosarcoma, we also analyzed 2 year PFS and OS in patients with
post- treatment β-hCG staining of ≥ 50% (n=2) compared to those
with negative or < 50% staining. Patients with post-treatment β-hCG
staining ≥ 50% had a median PFS of 6.1 months vs 19.2 months in
patients with β-hCG staining less than 50% (p = 0.03). Patients with
post-treatment β-hCG staining ≥ 50% also demonstrated a trend
towards shorter median OS of 17.1 months vs 19.2 months (Figure 3).
P16
Ten patients had diagnostic specimens available for P16 staining.
Of these 10 patients, 7 had post-treatment resection specimens
available with percent tumor necrosis detailed on final pathology
report. Five out of 7 patients had positive staining for P16 on pretreatment
diagnostic specimen. There was no statistically significant
relationship between P16 staining on pre-treatment diagnostic
osteosarcoma specimen and post-treatment percent tumor necrosis
on resection specimen. Patients with negative P16 staining on the
diagnostic specimen (n=3) did have a statistically significant lower
2 year PFS (0%, mean 10.9months) compared to those with positive
(n=7) P16 staining (2 year PFS 71%, mean 25.9 months), p=0.022.
There was a trend toward worse 2 year overall survival in patients with
P16 negative diagnostic specimens compared to patients with P16
positive tumors, 22% vs 86%, however this did not reach statistical
significance (p=0.25) (Figure 4).
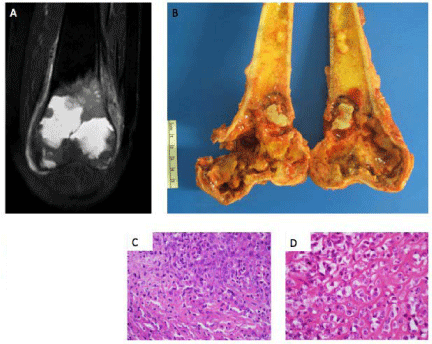
Figure 2
Figure 2
Index patient: A, MRI left femur. B, gross specimen. C, Diagnostic
open biopsy hematoxylin-eosin. D, Post-treatment hemoatoxylin-eosin.
Table 1
Discussion
Our index patient was a 30 year old woman who presented with
a left distal femur mass and an elevated serum β-hCG. Biopsy of the
distal femur mass revealed a high- grade sarcoma most consistent
with osteosarcoma with epithelioid features. Scattered tumor cells
within the infiltrate did stain positive for β-hCG. Staging scans
included CT chest which demonstrated two 2mm left lung nodules
that were too small to characterize and a whole body bone scan
which demonstrated increased activity in the distal left femur but no
other focal abnormal activity elsewhere to suggest metastatic disease.
Final staging of her tumor was stage IIa (T1N0M0G3). She initiated
neoadjuvant chemotherapy with doxorubicin, cisplatin and high-dose
methotrexate. Her β-hCG on C1D1 of therapy was 2288 mIU/mL
and was trended throughout chemotherapy with an initial decrease
to 1393 mIU/mL at week four and final pre-surgical β-hCG of 2417
mIU/mL at week 10. Restaging imaging prior to surgical resection
demonstrated stable 2 mm left upper lobe lung nodules and no evidence
of metastatic disease. MRI of the left femur however demonstrated
extension of the osteosarcoma of the distal femur into the knee joint
synovial soft tissues and an associated pathologic fracture that had
progressed since initial imaging. She proceeded to surgical resection
and underwent a left above knee amputation. Surgical pathology from
resection demonstrated a high-grade osteosarcoma measuring 12 x
6 x 6cm with extension though the lateral meta- epiphyseal cortex
into surrounding tissue and resulting in skin ulceration. Tumor
showed approximately 40% chemotherapeutic response. Margins
were negative. One lymph node was removed with no evidence of
sarcoma. Final pathology staging was pT2pN0Mx (Figure 2). Three
weeks after amputation, she presented to the emergency room with
cough and fever. β-hCG remained detectable in serum at 26.1 mIU/
mL. CT chest demonstrated a large multiloculated pleural effusion
and low density lung mass occupying the entire left hemithorax
with mass effect concerning for empyema, rapidly growing lung and
pleural based masses as well as new metastatic nodules in the right
lung. Left video assisted thoracoscopic surgery demonstrated a very
small amount of free pleural fluid and a white firm necrotic appearing
material was occupying much of the space. Pleural biopsy pathology
was positive for high grade sarcoma.
Chorionic gonadotropin is a hormone produced by trophoblast
that promotes growth of a developing embryo during pregnancy
or less commonly, promotes growth and invasion in gestational
trophoblastic disease [12]. Ectopic production of β-hCG by nontrophoblastic
tumors has been reported [6], however our review
of the literature found that the ectopic production of β-hCG by
osteosarcomas has only been recognized in several case reports and
small retrospective studies [8,13-18]. Masrouha et al. [7] reviewed
histopathology slides of thirty-two patients with osteosarcoma and
retrospectively stained the slides for β-hCG. Five of the thirty- two
patients’ specimens stained positive for β-hCG. In these five patients
there was a trend toward poor outcomes (which they define as initial
presence or development of metastatic disease or tumor recurrence)
and clinically more aggressive tumors (as defined by post-therapy
percent necrosis). While there is a trend towards worse outcomes in
patients whose tumor expresses β-hCG, more robust data is needed to
further characterize the significance of ectopic expression of β-hCG
by the tumor and outcomes. Despite the ununiformed expression
of β-hCG in osteosarcoma patients, it raises the question of the
potential use of this marker in a subgroup of patients. Intact human
chorionic gonadotrophin (hCG) is produced by normal placenta
and germ cell tumors while epithelial tumors typically produce the
free β subunit [19]. The intact heterodimeric hCG is part of normal
pregnancy, developmental signaling and tissue differentiation;
however Iles et al suggested that the free β subunit may have very
different effects on the secreting tumors, specifically anti-apoptotic
and pro-angiogenic influences. These properties may be related to the
structure of β- hCG which is a member of the cysteine knot growth
factor/TGFb superfamily and shares features similar to VEGF and
TGFβ [20]. Butler et al. [21] demonstrated an increase in a bladder
carcinoma cell population treated with β-hCG correlating with a
decrease in apoptotic bodies using MTT assay in a dose-dependent
fashion further supporting an anti-apoptotic effect of β-hCG. Several
investigators have demonstrated a correlation between VEGF, hCG
and angiogenesis in the placenta and developing ovarian follicles [22]
as well in tumor angiogenesis [23,24]. The direct role of β-hCG in
tumor angiogenesis remains to be more clearly defined.
There has also been further investigation into understanding
the role of β-hCG in chemoresistance. Sahoo et al. [24] examined
molecular pathways mediating continued tumor cell proliferation in
hCG exposed cells despite chemotherapy. Tumor cells pre-exposed
to hCG and then treated with chemotherapy (including 5-FU and
etoposide) demonstrated increased viability and increased rates of
proliferation compared to non-hCG exposed cells. They found the
mechanism of chemoresistance does not appear to be driven by one
mechanism alone but suggests a multifactorial explanation including
evasion of apoptosis and maintenance of cytokines associated with
tumorigenesis typically reduced with chemotherapy [25]. Our results
demonstrate worse clinical outcomes in patients with post-treatment
β- hCG tissue staining of ≥ 50% compared to those with negative or
< 50% staining as defined by decreased 2 year PFS and OS. While
we did not see a statistically significant influence of diagnostic
β-hCG tumor expression on outcomes, there was a trend towards
poorer outcomes in patients with any positive β-hCG on posttreatment
specimens. These results do raise the question if increased
expression of β-hCG by osteosarcoma tumors post-chemotherapy is
a marker or even mechanism of chemoresistance. Larger prospective
investigation is warranted to confirm post-treatment β-hCG staining
of osteosearcoma tumors as a prognostic tissue marker. We also note
that abrogation of the G1 cell-cycle checkpoint occurs in a variety
of malignancies and investigated the role of P16 in osteosarcoma on
clinical outcomes.
Cyclin-dependent kinase 4 inhibitor referred to as P16INK4a
(P16) is a major component of the G1 cell-cycle checkpoint, which
also includes the retinoblastoma protein (pRB) and cyclin D1
[26]. Inactivation of P16 or pRB proteins by mutation, deletion, or
promoter hypermethylation has been associated with continuous cell
proliferation in numerous malignancies, and loss of P16 expression
has been correlated with worse survival in osteosarcoma [9,10,27].
In a study conducted by Borys et al. [11] immunohistochemistry
staining for P16 was performed on 40 specimens from patients with
osteosarcoma. P16 expression correlated positively with post-therapy
percent tumor necrosis. In a similar study conducted in a pediatric
osteosarcoma population by Maitra et al, tissue from 38 patients
were stained for P16 expression [10]. Sixteen percent demonstrated
a loss of P16 expression and this absence significantly correlated with
decreased overall survival. We have confirmed similar findings in our
investigation with patients whose tumors had negative P16 staining
at diagnosis having a statistically significant lower 2 year PFS and
a trend towards shorter 2 year overall survival (22% vs 86%). We
recognize there are several limitations to our study including that
it was a retrospective analysis. P16 and β-hCG may be expressed
heterogeneously within a tumor and not completely represented on
the small biopsy specimen typically obtained to make the diagnosis
of osteosarcoma lending to potential for false positives or negatives.
In addition, our cohort size was smaller than anticipated due to
limited availability of tissue specimens, which in many instances were
obtained at outside hospitals prior to referral to our institution and
then returned after pathology review.
Conclusion
In conclusion, we found that almost half of osteosarcoma patients included in our cohort had tumors which expressed β-hCG at diagnosis and post neoadjuvant chemotherapy, including 25% of patients who had β-hCG staining of over 50% post neoadjuvant chemotherapy. The patients who had post-treatment β-hCG staining greater than or equal to 50% of tumors cells had a statistically significant decreased 2 year PFS and trend towards worse OS. This is similar to previous studies correlating positive β-hCG staining with worse outcomes, but more specifically defines significance in the post-treatment setting. This provides a prognostic marker unique to the individual tumor post-neoadjuvant therapy and we speculate this may be a marker of chemoresistance or even a mechanism with upregulation of β-hCG. Further investigation is needed in the prospective setting with a larger study population and consideration of additional markers including anti-apoptotic proteins and tumorigenic cytokines to better understand the role of β-hCG expression in osteosarcoma tumors. Our cohort size was too small to evaluate the significance of change in β-hCG expression from the diagnostic specimen compared to the post-treatment tissue. Our findings correlating negative P16 expression in tumors to more unfavorable clinical outcomes are similar to previous reports [9.11] and confirm P16 as a potential prognostic marker at diagnosis.
Acknowledgment
We would like to thank the DeBoer Family Research Initiative for funding this work.
References
- Howlander N NA, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, et al . SEER Cancer Statistics Review National Cancer Institute, Bethesda, Cancer Facts and Figures: American Cancer Society, Atlanta, GA. 2016.
- Bacci G, Longhi A, Versari M, Mercuri M, Briccoli A, Picci P. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer. 2006; 106: 1154-1161.
- Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002; 20: 776-790.
- Davis AM, Bell RS, Goodwin PJ. Prognostic factors in osteosarcoma: a critical review. J Clin Oncol. 1994; 12: 423-431.
- Braunstein GD, Vaitukaitis JL, Carbone PP, Ross GT. Ectopic production of human chorionic gonadotrophin by neoplasms. Ann Intern Med. 1973; 78: 39-45.
- Masrouha KZ, Khattab R, Tawil A, Abdallah A, Saghieh S, Haidar R, et al. A preliminary investigation of Beta-hCG expression in patients with osteosarcoma. J Bone Joint Surg Br. 2012; 94: 419-424.
- Oshrine BR, Sullivan LM, Balamuth NJ. Ectopic production of beta-hCG by osteosarcoma: a case report and review of the literature. J Pediatr Hematol Oncol. 2014; 36: e202-6.
- Bu J, Li H, Liu LH, Ouyang YR, Guo HB, Li XY, et al. P16INK4a overexpression and survival in osteosarcoma patients: a meta analysis. Int J Clin Exp Pathol. 2014; 7: 6091-6096.
- Maitra A, Roberts H, Weinberg AG, Geradts J. Loss of p16 expression correlates with decreased survival in pediatric osteosarcomas. Int J Cancer. 2001; 95: 34-38.
- Borys D, Canter RJ, Hoch B, Martinez SR, Tamurian RM, Murphy B, et al. P16 expression predicts necrotic response among patients with osteosarcoma receiving neoadjuvant chemotherapy. Hum Pathol. 2012; 43: 1948-1954.
- Cole LA, Dai D, Butler SA, Leslie KK, Kohorn EI. Gestational trophoblastic diseases: 1. Pathophysiology of hyperglycosylated hCG. Gynecol Oncol. 2006; 102: 145-150.
- Boss DS, Glen H, Beijnen JH, de Jong D, Wanders J, Evans TR, et al. Serum beta-HCG and CA-125 as tumor markers in a patient with osteosarcoma: case report. Tumori. 2011; 97: 109-114.
- Kalra JK, Mir R, Kahn LB, Wessely Z, Shah AB. Osteogenic sarcoma producing human chorionic gonadotrophin. Case report with immunohistochemical studies. Cancer. 1984; 53: 2125-2128.
- Leidinger B, Bielack S, Koehler G, Vieth V, Winkelmann W, Gosheger G. High level of beta-hCG simulating pregnancy in recurrent osteosarcoma: case report and review of literature. J Cancer Res Clin Oncol. 2004; 130: 357-361.
- Ordonez NG, Ayala AG, Raymond AK, Plager C, Benjamin RS, Samaan NA. Ectopic production of the beta-subunit of human chorionic gonadotropin in osteosarcoma. Arch Pathol Lab Med. 1989; 113: 416-419.
- Tuy BE, Obafemi AA, Beebe KS, Patterson FR. Case report: elevated serum beta human chorionic gonadotropin in a woman with osteosarcoma. Clin Orthop Relat Res. 2008; 466: 997-1001.
- Glass R, Asirvatham JR, Kahn L, Aziz M. Beta-human chorionic gonadotropin producing osteosarcoma of the sacrum in a 26-year-old woman: a case report and review of the literature. Case Rep Pathol. 2015; 897230.
- Iles RK, Delves PJ, Butler SA. Does hCG or hCGbeta play a role in cancer cell biology? Mol Cell Endocrinol. 2010; 329: 62-70.
- Lapthorn AJ, Harris DC, Littlejohn A, Lustbader JW, Canfield RE, Machin KJ, et al. Crystal structure of human chorionic gonadotropin. Nature. 1994; 369: 455-461.
- Butler SA, Ikram MS, Mathieu S, Iles RK. The increase in bladder carcinoma cell population induced by the free beta subunit of human chorionic gonadotrophin is a result of an anti-apoptosis effect and not cell proliferation. Br J Cancer. 2000; 82: 1553-1556.
- Demir R, Kayisli UA, Seval Y, Celik-Ozenci C, Korgun ET, Demir-Weusten AY, et al. Sequential expression of VEGF and its receptors in human placental villi during very early pregnancy: differences between placental vasculogenesis and angiogenesis. Placenta. 2004; 25: 560-572.
- Arrieta O, Michel Ortega RM, Angeles-Sanchez J, Villarreal-Garza C, Aviles-Salas A et al. Serum human chorionic gonadotropin is associated with angiogenesis in germ cell testicular tumors. J Exp Clin Cancer Res. 2009; 28: 120.
- Li D, Wen X, Ghali L, Al-Shalabi FM, Docherty SM, Purkis P, et al. hCG beta expression by cervical squamous carcinoma--in vivo histological association with tumour invasion and apoptosis. Histopathology. 2008; 53: 147-155.
- Sahoo S, Singh P, Kalha B, Singh O, Pal R. Gonadotropin-mediated chemoresistance: Delineation of molecular pathways and targets. BMC Cancer. 2015; 15: 931.
- Bartek J, Lukas J, Bartkova J. Perspective: defects in cell cycle control and cancer. J Pathol. 1999; 187: 95-99.
- Serra M, Scotlandi K, Reverter-Branchat G, Ferrari S, Manara MC, Benini S, et al. Value of P-glycoprotein and clinicopathologic factors as the basis for new treatment strategies in high-grade osteosarcoma of the extremities. J Clin Oncol. 2003; 21: 536-542.