Research Article
Neoadjuvant Immunotherapy in High Risk Patients with Cutaneous Melanoma: A Novel Approach
Elias EG*
Former Professor of Surgery & Oncology, University of Maryland School of Medicine, USA
*Corresponding author: E. George Elias, Former Professor of Surgery & Oncology, University of Maryland School of Medicine, 1214 Merediths Ford Road, Towson, Maryland 21286-1320, USA
Published: 27 Aug, 2016
Cite this article as: Elias EG. Neoadjuvant Immunotherapy
in High Risk Patients with Cutaneous
Melanoma: A Novel Approach. Clin
Oncol. 2016; 1: 1060.
Abstract
Cutaneous melanoma is an immunogenic tumor, but it seems to be very heterogeneous. Utilizing
patient own tumor, before it excision, as the source for tumor-specific antigens, intratumoral
administration of low dose of GM-CSF weekly in dermal and subdermal metastases did result in over
50% response rate. Failure to establish complete tumor response (CR), low weekly dose of IL-2 was
substituted and resulted in CR. Therefore, it seemed that some melanoma lesions did therapeutically
respond to intratumoral GM-CSF therapy, while other lesions required Intratumoral IL-2. Each
of these two cytokines has different mechanism of action that may complement one another.
Therefore, sequential administration of GM-CSF followed by IL-2, once at the primary site of deeply
invasive primary melanoma, one week prior to its resection (Neoadjuvant Approach), did induce
massive antitumor immune response at the injection sites. Such an immune response did result in
complete tumor necrosis with massive histiocytosis. In addition, there was an overexpression of a
great number of immune cells at the injection sites as well as in some regional lymph nodes. This
autologous approach seemed to overcome tumor heterogeneity. The overall duration of response
ranged from 31- over 60 months to the last date of contact. In conclusion, in vivo autoimmunization
of melanoma sites (prior to its excision) by intratumoral administration of these two cytokines
seemed to induce an immense antitumor response without major side effects, and such immune
response was transmitted via the lymphatics. Such an approach seemed to prolong patient survival.
Keywords: Preoperative intratumoral cytokine therapy; Survival benefits
Abbreviations
GM-CSF: Granulocyte-Macrophage Colony Stimulating Factor (also known as Leukine) manufactured by Sanofi-Aventis Corporation; IL-2: Interleukin-2 (also called Aldesleukin) manufactured by Chiron Corporation
Introduction
Justification for the new approach
Tumor heterogeneity: Cutaneous melanoma is an immunogenic tumor as it expresses various
melanoma-specific antigens. However, it seems to be very heterogeneous both clinically and
biologically. Clinically; some melanomas develop in sun exposed skin areas, while others develop in
none exposed sites. Furthermore, melanomas can present with various amount of pigmentation that
ranges from black to none pigmented lesions known as “Amelanotic Melanoma”. In addition, some
melanomas metastasize to the regional lymph nodes initially then systemically, while others do
metastasize directly to the viscera and the brain. Biologically, melanoma expresses different specific
antigens and has diverse genetic profiles among different patients. To overcome such heterogeneity,
tumor-specific and autogenic therapeutic approach, for each patient, could be essential to obtain an
antitumor immune response. It has been shown that patients with resected metastatic melanoma
who have melanoma-specific infiltrating lymphocytes (TILs) in the resected metastases have
statistically better survival than those who have melanoma-specific T cells in the peripheral blood
[1]. Furthermore, the higher the number of TILs at the primary sites of melanoma carries better
prognosis [2]. Therefore, the activation of these cells at the tumor site is a logic approach to obtain
antitumor immune response.
Adjuvant therapy; past and present: Early trials with systemic adjuvant therapy, administered
after resection of the melanoma, included non-specific immune stimulants such as BCG (bacillus
Calmette Guerin), Corynebacterium parvum, levamisole or combinations of these agents with
and without chemotherapy with decarbazine (DTIC) revealed no significant impact on the
disease [3]. In addition, adjuvant vaccines trials were ineffective and
sometimes harmful except with autologous melanoma vaccine [4].
High dose postoperative systemic administration of interferon α-2b
as an adjuvant therapy for one year did initially show significant
improvement in disease-free and overall survival [5]. However, the
overall survival benefit was not sustained overtime [6].
The neoadjuvant approach: To utilize patients’ own tumor as the
source for melanoma-specific antigens, prior to its excision, and to
activate the local immune response at the tumor site could overcome
such heterogeneity.
In the meantime, two cytokines have shown activity in the
management of dermal metastatic melanoma. These included
granulocyte-macrophage colony stimulating factor (GM-CSF) and
intrleukin-2 (IL-2).
GM-CSF is a multifunctional molecule administered as a single
agent in dermal metastases can increase the number and activation
of autologous dendritic cells (DCs), T cell infiltrate at the tumor site
particularly helper cells and increases the expression of IL-2 receptors
(IL-2R) on some T lymphocytes. DCs are very efficient antigen
presenting cells (APCs) capable of processing tumor antigens and
present the processed antigens by crosstalk to T lymphocytes in the
context of major histocompatibility class I and II molecules. DCs are
also rich in co-stimulatory factors such as B7-1 and B7-2 which are
needed to complete the second immune signal to T lymphocytes which
become committed to specific immune response. Its intratumoral
administration at doses of 10-80 μg has shown its biological effects but
without major clinical benefit [7,8]. However, when the doses were
increased to 400-500 μg daily for 4-5 consecutive days and repeated
every 21-28 days, it has given excellent clinical responses but with
some side effects [9,10]. On the other hand, IL-2 is a glycoprotein
immune modulator [11]. Its intratumoral administration as a single
agent in in-transit metastatic melanoma at doses ranging from 0.6-6.0
million IU, 2-3 times per week or with escalating doses has resulted in
complete tumor response (CR) in two thirds of the patients, but with
grade I and II toxicity especially at the higher doses [12-14].
To investigate the effect of intratumoral administration of low
dose GM-CSF and IL-2 in melanoma lesions, patients with dermal
and subdermal lesions were chosen as these lesions were accessible
to intratumoral therapy and could be repeatedly inspected, palpated
and easily biopsied to confirm the effect of therapy. Low doses of both
cytokines were utilized to avoid toxicity. GM-CSF was administered
first, and in case of failure to obtain CR, intralesional IL-2 was
substituted.
Patients and Methods
Patients with dermal and subdermal metastases, including those
with in-transit metastases were initially studied. This was regardless
to the extent of the disease, anatomic site of involvement or previous
therapy. The study patient did not receive any other anticancer
therapy while on the study. None of the lesions were evaluated for
their tumor antigenicity or genetic expressions. However, the treated
tumor sites had to have pathological confirmation for the presence of
melanoma [15].
Each patient received intratumoral low dose of GM-CSF (500
microgram) once/week. Those who failed to respond to GM-CSF
therapy were managed by intratumoral therapy with low dose IL-2 (11
million IU) weekly. All these patients were previously treated for their
metastases by various methods including repeated local excisions,
intratumoral BCG, and hyperthermic isolated limb perfusion with
melphalan, limb infusion, radiation therapy, systemic therapy and
combinations of the above.
Among them were two patients who were failure to postoperative
systemic adjuvant therapy with higher doses of GM-CSF and IL-2
administered subcutaneously. GM-CSF was given first at 125 μg/
m2/day for 14 consecutive days followed by IL-2 at 9 million IU/m2/
day for 4 days, repeated every month for 2 years. They developed
in-transit metastases; one within a year of initiating such adjuvant
therapy, and the other 3 months after completing 2 years of the
adjuvant therapy. Another two patients had distant metastases with
palpable subdermal metastatic lymph nodes were included; one had
palpable supraclavicular metastases (site of intratumoral therapy)
with distant metastases to left iliac lymph node, and the other had
palpable metastatic lymph nodes under the skin of the anterior
axillary fold with lung metastases. Each received intratumoral therapy
at the palpable masses.
A second group of patients consisted of those with invasive
primary skin lesions with satellitosis or in-transit metastases and
regional lymph node metastases, i.e., stage III disease, but remained
to be surgical candidates with very guarded prognosis. Each received
preoperative intratumoral sequential injection of 500 μg GM-CSF at
the primary site and at the dermal metastases on day # 1, followed by
11 million IU at the same sites on day #2, just one week before the
planned surgical resection.
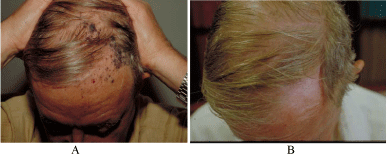
Figure 1
Figure 1
A photograph of melanoma of the scalp:
A. Before intratumoral therapy: The patient presented with multiple in-transit
metastases with unidentified primary site among the metastases.
B. After intratumoral therapy with low dose GM-CSF administered weekly for
4 weeks: Note the complete clinical response, proven pathologically by rebiopsy.
The patient had no surgical resection or other treatment and is alive
free of disease for over 5 years.
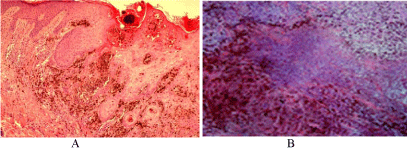
Figure 2
Figure 2
Pathological confirmation of the response:
A. Before intratumoral therapy, from the initial biopsy of the primary site.
Please notice the depth of invasion.
B. One week after the administration of neoadjuvant therapy with GM-CSF
and IL-2. Notice the complete tumor necrosis and massive histiocytosis at the
injection site (primary site). X400, H & E staining.
Results
There was over 50% CR to intratumoral therapy with GM-CSF,
an example can be seen in Figure 1. All failures to GM-CSF therapy
were successfully treated by intratumoral IL-2. This included the two
patients who were previously treated with systemic adjuvant therapy.
The other two patients with distant metastases, both responded to
intratumoral cytokine therapy at the injection sites. One had CR at
the distant metastatic iliac lymph node after receiving intratumoral
therapy in the supraclavicular lymph nodes. Some of the treated
sites with either cytokine were biopsied 6-8 weeks after cessation of
therapy and the histopathological examination revealed no residual
tumor cells or mononuclear cell infiltrates.
However, patients with large sclerotic coalesced skin lesions
of over 3 cm each but without evidence of metastases failed to
show response to either cytokine therapy. Furthermore, some of
the previously treated patients who were successfully treated by
intratumoral cytokines developed recurrences at 12-22 months in
none of the treated sites with intratumoral cytokines.
It seemed that some melanoma lesions did respond to
intratumoral administration of GM-CSF while other lesions require
IL-2 intratumoral therapy. As each of these 2 cytokines has different
mechanism of action that could complement one another, sequential
administration of GM-CSF followed by IL-2 could be more effective.
The next group of patients, who were not previously treated and
each had stage III melanoma, but remained to be surgical candidates
but with very guarded prognosis. Each was managed by neoadjuvant
immunotherapy that consisted of intratumoral administration of GMCSF
followed by IL-2 on two consecutive days, one week before the
surgical resection. The clinical response could not be assessed because
of the local reaction in the short time period of one week between
the injection to the surgical resection. However, the histopathological
examination of the resected tissues revealed complete tumor necrosis
with massive histiocytosis at the injection sites, Figure 2. On the
other hand, the enlarged regional lymph nodes harbored metastases.
This was not a surprise, because of the short duration between the
injections and the resection, after a single course of therapy. These
patients were alive and free of disease for over 5 years.
Immunohistochemical studies were performed on the
resected tissue, one week after the administration of neoadjuvant
therapy, utilizing commercially available antibodies. This showed
overexpression of several immune cells that included CD3+ (total
T cells), CD8+ (cytotoxic T cells which are the actual tumor killer
cells), CD4+ (helper cells) and CD83+ (mature dendritic cells) [16].
Figure 3 shows the effect of neoadjuvant therapy on CD8+ cells at
the primary site. In addition, these immune cells were also detected
in some of the resected regional lymph nodes, an example of CD8+
cells can be seen in Figure 4. It should be noted that all these immune
cells were autologous to each patient. These patients are alive free of
disease for over 5 years.
Discussion
It should be realized that these two cytokines have no direct
cytotoxic effects on tumor cells. Their function is mediated through
the induction and activation of immune cells in vivo.
Intratumoral therapy with low dose GM-CSF and IL-2 was
safe, i.e., did not cause any tumor dissemination, well tolerated and
seemed to be more effective than the more frequent intratumoral
administration of higher doses of either cytokine. In addition, none
of the treated patients had any systemic side effects in the form of
fever, chills, fatigue, rash or any significant changes in their CBC or
serum chemistry including the LDH. The only side effect was local
skin reaction at the injection sites.
The neoadjuvant approach was effective and relatively cheaper
than any futuristic adjuvant therapeutic approach such as the use of
anti-cytotoxic T lymphocyte associated antigen-4 (anti CTLA-4) or
anti-program cell death and its primary legend (anti PD-1 and PDL-
1) which showed some survival benefits in patients with metastatic
melanoma [17,18]. A recent adjuvant study by the European group
proved this point utilizing anti CTLA-4 (Ipilimumab) after surgical
resection of stage III melanoma showed some early success but
with 48% recurrence rate at a median of 2.7 years, with grade 3 and
4 immune related adverse events that required the discontinuation
of the therapy in 52% of the patients [19]. Intratumoral GMCSF
alone resulted in over 50% CR, and failure to secure CR was
successfully rescued by IL-2 therapy. This again shows heterogeneity
of melanoma. Therefore, it seemed that some melanoma lesions did
respond to the activation of dendritic cells by intratumoral GM-CSF,
while other lesions required the activation of the cytotoxic T cells
by IL-2. Therefore, the sequential administration of both cytokines
seemed to be justified.
Intratumoral therapy with these cytokines utilized each patient
own tumor as the source for tumor-specific antigens. It was effective
in metastatic lesions as well as in primary invasive melanoma.
The only failures were large sclerotic skin lesions, probably due to
the large tumor load and the sclerotic nature of the lesions from
previous therapy that could not be handled immunologically [15].
Hypothetically, such lesions could be excised followed by cytokines
injections at the resection margins for two to three weeks prior to
skin grafting.
It was of interest to notice the absence of any residual tumor
cells or mononuclear cell infiltrates 6-8 weeks after complete clinical
response to intratumoral cytokine therapy. This could indicate an
immense autologous antitumor immune response to the therapy with
complete washout of the local effects over such period of time. This
was further confirmed by histopathological evaluating the resected
tissue, one week after preoperative intratumoral administration of
both GM-CSF and IL-2. This did clearly show an efficient anti-tumor
response within days after the administration of both cytokines at
the injection (tumor) sites, as seen in Figure 2. In addition, there was
overexpression of various immune cells at the injection sites that
included cytotoxic T cells (CD8+) as seen in Figure 3, as well as helper
cells (CD4+) and mature dendritic cells (CD83+). Furthermore, these
immune cells were also identified in some regional lymph nodes
that contained no metastases. An example can be seen in Figure 4.
This might suggest that such an immune response was taken-up
by the lymphatics and could have possibly eliminated early micrometastases
in a patient with stage IIIC disease. Such findings confirm
other reports that the administration of GM-CSF near the biopsy
site of primary cutaneous melanoma can increase the number and
activation of dendritic cells and tumor-specific cytotoxic T cells in
sentinel lymph node [20,21].
The route of the administration of a vaccine can be a critical
variable in determining the outcome of an immune response. In an
animal model, when a vaccine with naked antigen-encoding RNA is
being administered in the skin, subcutaneous tissue or near a lymph
node, no significant immune response has been noted. However,
when this vaccine is administered in a lymph node, it elicited potent
prophylactic and therapeutic antitumor immunity [22]. Therefore,
it was no surprise to obtain CR in two patients who failed systemic
adjuvant therapy with both cytokines but responded to intratumoral
therapy with low doses of the same cytokines.
Furthermore, the two patients with distant metastases who had
palpable subdermal metastatic lymph nodes had CR at the injection
sites, with one of them had CR in the distant metastatic iliac lymph
node after intra-lymphatic cytokine therapy at the supraclavicular
metastatic lymph nodes. This could suggest a possible role for intralymphatic
injection of these cytokines. However, while intralesional
therapy could initiate an antitumor immune response in patients with
distant metastases, it would need systemic support as the injected
sites (source of antigen) did dissolve after the initial intratumoral
therapy, and therefore such therapy could not be continued. On the
other hand, it could be speculated that patients with limited distant
metastases could be treated with intratumoral cytokine therapy
utilizing sonographic or CT control.
Recurrences did occur at 12 and 22 months in previously treated
patients. This could be due to the development of some tolerance
from previous systemic adjuvant therapy with both cytokines, and
secondary to the use of immune suppressive therapy by chemotherapy
and radiotherapy. Such recurrences could have been managed by
repeated intralesional cytokine therapy at the recurrence sites rather
than by surgical excisions.
The durable response in primary melanoma lesions could suggest
a role for preoperative intratumoral administration of both cytokines
as a neoadjuvant therapy in high risk primary melanoma, and newer
prospects of targeted cancer therapy. Furthermore, this study showed
a promising anticancer therapeutic strategy which could be reliant on
formation of an immune microenvironment at the tumor site, and it
may be applicable to other solid malignancies.
This specific autologous approach had its limitation as it should
not be used in infected lesions or with allogenic antigens as it may
result in an immune deviation. An example can be seen in two reports
of active immunization with two vaccines; one with multi-peptides
and the other with allogenic whole melanoma cells +3 peptides in
combination with GM-CSF that resulted in a negative outcome
[23,24]. The authors blamed such negative results on the use of GMCSF,
but the fact is that their antigens that were used, whether peptides
or allogenic cells, did not express patients’ own tumor antigens and
as a result the induced immune response by GM-CSF was directed to
the administered antigens.
The response seen with intratumoral therapy with these two
cytokines targeted the whole tumor cells that could be regardless
to its antigenic or genetic profiles. It can also replace the repeated
surgical excision of local recurrences in-transit metastases. It should
be pointed out that combined (mixed) administration of these two
cytokines has not been tried.
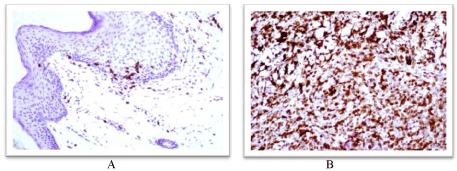
Figure 3
Figure 3
An example of the immunohistochemical response to neoadjuvant
therapy showing the effect on the cytotoxic T cells (CD8+):
A. Before the neoadjuvant therapy, from the biopsy of the primary lesion.
Note the few number of CD8+ cells (reddish cells) at the epidermal/dermal
junction.
B. One week after the administration of neoadjuvant therapy, from resected
tissue of the primary site of the same patient. Notice the overexpression of
CD8+ cells. X400, using commercially available antibody.
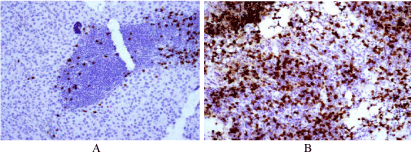
Figure 4
Figure 4
Showing the immunohistochemical effect of neoadjuvant therapy,
on the regional Lymph nodes
A. Lymph node of a patient who did not receive preoperative neoadjuvant
therapy. Note the low number of CD8+ cells (reddish cells).
B. Lymph node of a patient who receive preoperative neoadjuvant therapy.
Please note the overexpression of CD8+ cytotoxic T cells one week after
therapy. X400, using commercially antibody.
Conclusions
Intratumoral administration of GM-CSF and IL-2 prior to the surgical resection of the melanoma lesions as a Neoadjuvant Immunotherapy is non-toxic and effective in the management of patients with dermal and subdermal metastases as well as in patients who present with high risk primary melanoma. These new findings warrant the initiation of prospective controlled randomized studies. This approach may be applicable to other small solid malignancies.
References
- Haanen JB, Baars A, Gomez R, Weder P, Smits M, de Gruijl TD, et al. Melanoma-specific tumor-infiltrating lymphocytes but not circulating melanoma-specific T cells may predict survival in resected advanced-stage melanoma patients. Cancer Immunol Immunother. 2006; 55: 451-458.
- Azimi F, Scolyer RA, Rumcheva P, Moncrieff M, Murali R, McCarthy SW, et al. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol. 2012; 30: 2678-2683.
- Eggermont AM, Gore M. Randomized adjuvant therapy trials in melanoma: surgical and systemic. Smin Oncol. 2007; 34: 509-515.
- Berd D, Maguire HC Jr, Schuchter LM, Hamilton R, Hauck WW, Sato T, et al. Autologous hapten-modified melanoma vaccine as postsurgical adjuvant treatment after resection of nodal metastases. J Clin Oncol. 1997: 15; 2359-2370.
- Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH. Interferon α-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996; 14: 7-17.
- Kirkwood JM, Ibrahim JG, Sondak VK, Richards J, Flaherty LE, Ernstoff MS, et al. High and low dose interferon alfa-2b in high-risk melanoma: first analysis of intergroup trial E1690/S9111/C9190. J Clin Oncol. 2000; 18: 2444-2458.
- Si Z, Hersey P, Coats AS. Clinical responses and lymphoid infiltrates in metastatic melanoma following treatment with intralesional GM-CSF. Melanoma Res. 1996; 6: 247-255.
- Nasi ML, Lieberman P, Busam KJ, Prieto V, Pangeas KS, Lewis JJ, et al. Intradermal injection of granulocyte-macrophage colony-stimulating factor (GM-CSF) in patients with metastatic melanoma recruits dendritic cells. Cytokines Cell Mol Ther. 1999; 5: 139-144.
- Vaquerano JE, Cadbury P, Tressler P, Sagebiel R, Leong SPL. Regression of in-transit melanoma of the scalp with intralesional recombinant human granulocyte-macrophage colony-stimulating factor. Arch Dermatol. 1999; 135: 1276-1277.
- Hoeller C, Jansen B, Heere-Ress E, Pustelnik T, Nossbacher U, Schlagbauer-Wadl H, et al. Perilesional injection of r-GM-CSF in patients with cutaneous melanoma metastases. J Invest Dermatol. 2011; 17: 371-374.
- Medzhitov R, Janeway C Jr. Innate immune recognition: mechanisms and pathways. Immunol Rev. 2000; 173: 89-97.
- Radny P, Caroli UM, Bauer J, Paul T, Schlegel C, Eigentler TK, et al. Phase II trial of intralesional therapy with interleukin-2 in soft-tissue melanoma metastases. Br J Cancer. 2003; 89: 1620-1626.
- Dehesa LA, Vilar-Alejo J, Valeron-Almazan P, Carretero G. Experience in the treatment of cutaneous in-transit melanoma metastases and satellitosis with intralesional interleukin-2. Actas Dermosifiliogr. 2009; 100: 571-585.
- Weide B, Derhovanessian E, Pflugfelder A, Eigentler TK, Radny P, Zelba H, et al. High response rate after intratumoral treatment with interleukin-2: results from a phase 2 study in 51 patients with metastasized melanoma. Cancer. 116: 4139-4146.
- Elias EG, Sharma BK. Melanoma vaccines, revisited: a review, update. Gital Dermatol Venereol. 2014; 149: 711-717.
- Cao W, Lee SH, Lu J. CD 83 is performed inside monocytes, macrophages and dendritic cells, but it is only stably expressed on activated dendritic cells. Biochem J. 2005; 385: 85-93.
- Haanen JB. Immunotherapy of melanoma. EJC Suppl. 2013; 11: 97-105.
- Eggermont AM. Adjuvant therapy in high-risk melanoma. EJC Suppl. 2013; 11: 106-108.
- Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R,Wolchok JD, Schmidt H, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC18071): a randomised, double-blinded phase 3 triel. Lancet Oncol. 2015; 16: 522-530.
- Vuylsteke RJ, Molenkamp BG, Gietema HA, Gietema HA, van Leeuwen PAM, Vos W, et al. Local administration of granulocyte/macrophage colony-stimulating factor increases the number and activation state of dendritic cells in sentinel lymph node of early-stage melanoma. Cancer Res. 2004; 64: 8456-8460.
- Vuylsteke RJ, Molenkamp BG, van Leeuwen PAM, Meijer S, Wijnands PGJTB, Haanen JB, et al. Tumor-specific CD8+ T cell reactivity in sentinel lymph node of GM-CSF-treated stage I melanoma patients is associated with high myeloid dendritic cell content. Clin Cancer Res. 2006; 12: 2826-2833.
- Kreiter S, Selmi A, Diken M, Koslowski M, Britten CM, Huber C, et al. Intranodal vaccination with naked antigen-encoding RNA elicits potent prophylactic and therapeutic antitumoral immunity. Cancer Res. 2010; 70: 9031-9040.
- Slingluff CL, Petroni GR, Olson WC, Smolkin ME, Ross MI, Haas NB, et al. Effect 0f granulocyte/macrophage colony-stimulating factor on circulating CD8+ and CD4+ T-cell response to a multipeptide melanoma vaccine: outcome of a multicenter randomized trial. Clin Cancer Res. 2009; 15: 7036-7044.
- Faries MB, Hsueh EC, Ye X, Hoban M, Morton DL. Effect of granulocyte/macrophage colony-stimulating factor on vaccination with an allogenic whole-cell melanoma vaccine. Clin Cancer Res. 2009; 15: 7029-7035.