Research Article
Can High-Grade Prostate Cancer (Gleason 8-10) be Cured with Definitive Local Therapy without Hormone Suppression? Disease Control and Survival Outcomes after Up-Front Radical Prostatectomy in Patients with High-Grade Clinically Localized Disease
Mitchell DL1, Russo JK2, Mott SL3, Tracy CR4, Smith MC1, Buatti JM1 and Watkins JM1*
1Department of Radiation Oncology, University of Iowa, USA
2Bismarck Cancer Center, USA
3Holden Comprehensive Cancer Center, University of Iowa, USA
4Department of Urology, University of Iowa, USA
*Corresponding author: John M. Watkins, Department of Radiation Oncology, University of Iowa, Carver School of Medicine, 200 Hawkins Drive, Iowa City, Iowa 52242, USA
Published: 25 Aug, 2016
Cite this article as: Mitchell DL, Russo JK, Mott SL,
Tracy CR, Smith MC, Buatti JM, et
al. Can High-Grade Prostate Cancer
(Gleason 8-10) be Cured with Definitive
Local Therapy without Hormone
Suppression? Disease Control and
Survival Outcomes after Up-Front
Radical Prostatectomy in Patients
with High-Grade Clinically Localized
Disease. Clin Oncol. 2016; 1: 1057.
Abstract
Background: High-grade prostate cancer (HGPC) is associated with an aggressive clinical course
and poor outcomes, thus testosterone-suppressive hormone therapy (HT) frequently accompanies
definitive local therapy. Selected HGPC patients who undergo radical prostatectomy (RP) without
HT enjoy long-term disease control; however, the prognostic factor selection for this approach
remains to be identified.
Methods: A retrospective study of men diagnosed with biopsy-proven, clinically localized Gleason
(GS) 8-10 adenocarcinoma managed with primary RP from 2003 through 2010 was undertaken.
Patient-, tumor-, and treatment-related factors were analyzed for association with biochemical
(PSA) relapse-free surviva (bRFS), employing Cox proportional hazard regression.
Results: Among the 96 patients with HGPC who underwent RP, 69 met eligibility criteria. Median
age was 62 years (range, 48-75) and median pre-RP PSA was 7.1 ng/mL (3.5-64.9) with highest GS at
biopsy of 8, 9 and 10 for 41, 26 and 2 patients respectively. Highest GS at RP was <7, 8, and >9 for 23,
17, and 29 patients, respectively. Extraprostatic extension, involved surgical margin, seminal vesicle
invasion, and lymph node involvement were identified in 32, 33, 18, and 6 patients, respectively,
with adjuvant radiotherapy delivered to 5 patients immediately post-RP. At median follow-up of
67.3 months (2.7-141.2), 40 patients had disease recurrence and 8 patients died (6 cancer-specific).
Five-year bRFS and overall survivals were 39% (95% CI, 27-51%) and 87% (75-93%), respectively.
Primary grade and overall GS at RP, involved surgical margin, seminal vesicle involvement, nodal
involvement, and elevated initial post-prostatectomy PSA were significantly associated with bRFS
at univariate analysis, with primary grade at RP (HR=1.80; p<0.01) and post-RP PSA (HR=4.64;
p<0.01) significant at multivariable analysis.
Conclusions: HGPC is associated with high rates of early disease recurrence. Following RP without
systemic therapy, high primary grade and detectable initial post-RP PSA (>0.1 ng/mL) were
independently associated with worse bRFS.
Keywords: Prostate cancer; Radical prostatectomy; Survival analysis; Radiotherapy; Systemic
therapy
Introduction
Clinically localized high-grade prostate cancer (HGPC) is characterized by higher rates of cancerspecific
death relative to low or intermediate grade [1,2]. This is primarily a result of subsequent
manifestation of metastatic disease [1-3]. As a result of this, multidisciplinary consensus guidelines4
advocate for radiation therapy with long-term androgen deprivation therapy as curative-intent
interventions for HGPC, with local therapies (including brachytherapy and radical prostatectomy,
RP) considered alternative options [4]. These recommendations are
due, in part, to evidence from randomized trials demonstrating an
overall survival benefit for the addition androgen deprivation therapy
(“hormone therapy,” HT) to radiation therapy (RT) over RT alone
[5-7]. Despite this, several series have demonstrated long-term
disease control and survival after primary RP (with or without RT)
or primary RT (particularly with brachytherapy boost) in selected
populations of patients with HGPC [8-12]. Such approaches may
permit avoidance of the adverse metabolic effects of prolonged HT
[13]; however, identification of which HGPC patient subset(s) may
be safely managed with such an approach remains to be determined.
The objectives of the current investigation are to describe mature
disease control and survival outcomes for a population of patients
diagnosed with Gleason score (GS) ≥8 prostate cancer at biopsy who
were managed with local therapy alone. Secondary aims include
analysis for factors associated with disease control and survival,
in order to identify subset(s) who may potentially be cured by this
approach.
Table 1
Patients and Methods
Following Institutional Review Board (IRB) approval at the study
institutions, a research database was created from existing medical
records and quality assurance databases. Data collected included
demographic, tumor staging, treatment and outcome variables. The
study population included males with prostate adenocarcinoma
whose highest GS at biopsy was ≥8, without clinical or radiographic
evidence of suspicious regional lymph nodes or any distant metastases.
Patients with pre- or immediate post-operative (non-salvage) HT,
biopsy GS ≤7, or insufficient follow-up (defined as PSA follow-up
<12 months post-RP) were excluded. Follow-up consisted of PSA at
least every 3-6 months for 5 years, and annually thereafter. If patients
had PSA failure or clinical symptoms suggestive of relapse, re-staging
imaging was performed at the discretion of the primary urologist.
Decisions regarding salvage or intervention were also determined by
the managing urologist and oncologist.
The primary outcome for this retrospective analysis was PSA
(biochemical) relapse-free survival (bRFS); defined as PSA >0.1 ng/
mL and rising, clinical or radiographic evidence of recurrence, or
upon initiation of salvage therapy. Stable or oscillating PSA ≤0.1 were
identified but not considered events for bRFS. Overall survival (OS)
was measured from date of prostatectomy to date of death or last
clinical follow-up.
Statistical analysis
Survival probabilities were estimated and plotted using the
Kaplan-Meier method. Estimates, along with 95% pointwise
confidence intervals, were reported. Cox proportional hazards
regression was used to assess the effects of clinicopathologic variables.
Using a stepwise selection procedure, variables significantly associated
with bRFS at the univariate level were considered for inclusion in the
multivariable model. Estimated effects of predictors are reported as
hazard ratios (HR) along with 95% confidence intervals (C.I.). All
statistical testing was two-sided and assessed for significance at the
5% level using SAS, version 9.3 (SAS Institute, Cary, NC).
Table 2
Results
Between 2003 and 2010, 96 patients with GS ≥8 prostate cancer
underwent RP, of whom 69 were eligible for the present analysis.
Reasons for ineligibility were pre and/or post-RP hormone therapy
(n=11), non-curative or aborted prostatectomy (7), insufficient
follow-up (6), pre-RP chemotherapy (1), absence of cancer in biopsy
specimen at review (1), and metastatic disease at diagnosis (1). The
median age at diagnosis was 62 years (range, 48-75) and average PSA
at biopsy was 7.1 ng/mL (3.5-64.9). Detailed patient demographics,
pre-operative staging and tumor characteristics are outlined in Table
1. At RP, GS was <7, 8, and >9 for 23, 17, and 29 patients, respectively.
Extraprostatic extension, involved surgical margin, seminal vesicle
invasion, and lymph node involvement were identified in 32, 33, 18,
and 6 patients, respectively, with adjuvant radiotherapy delivered to 5
patients immediately following RP (Table 2).
At a median follow-up of 67.3 months (2.7-141.2), 40 patients
(58%) experienced disease recurrence (all initially detected by rising
PSA) and 8 patients had died (6 cancer-specific). The estimated
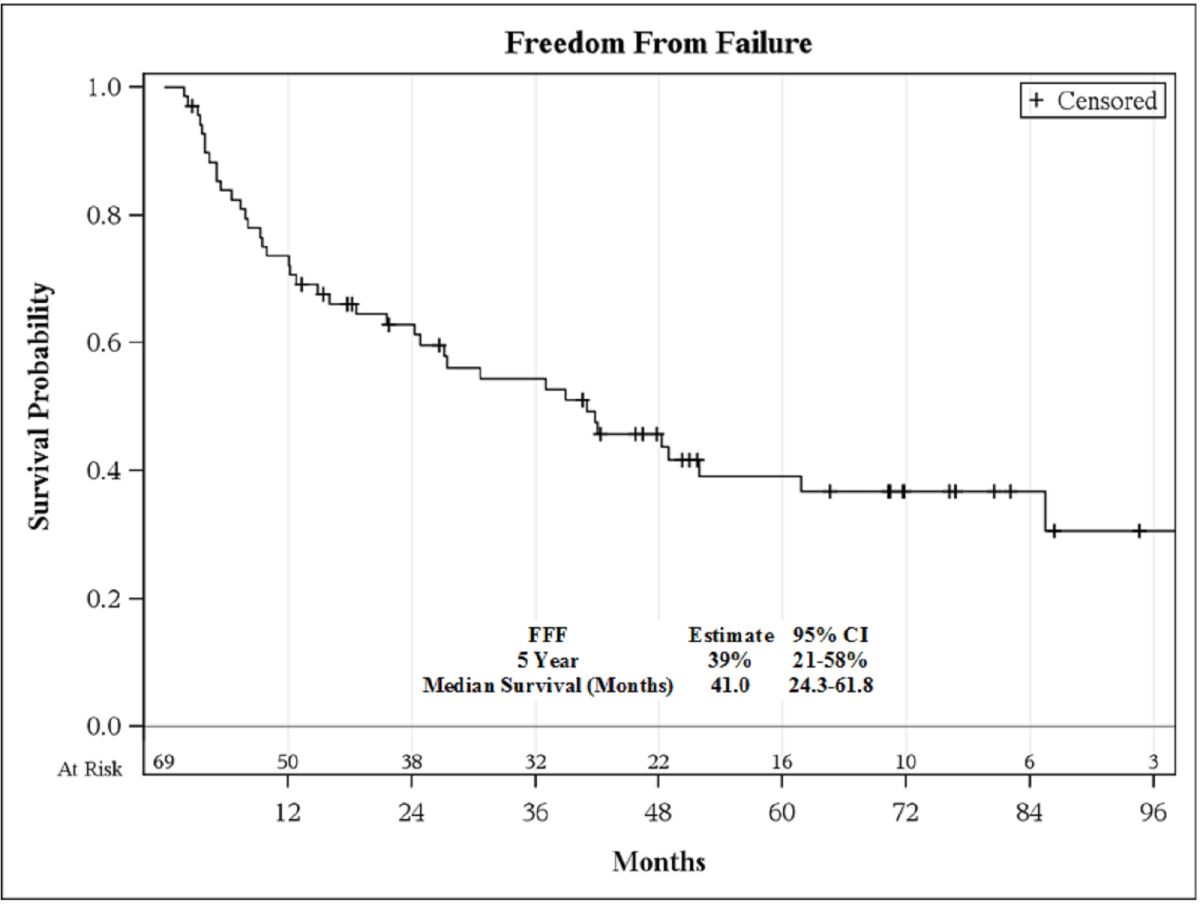
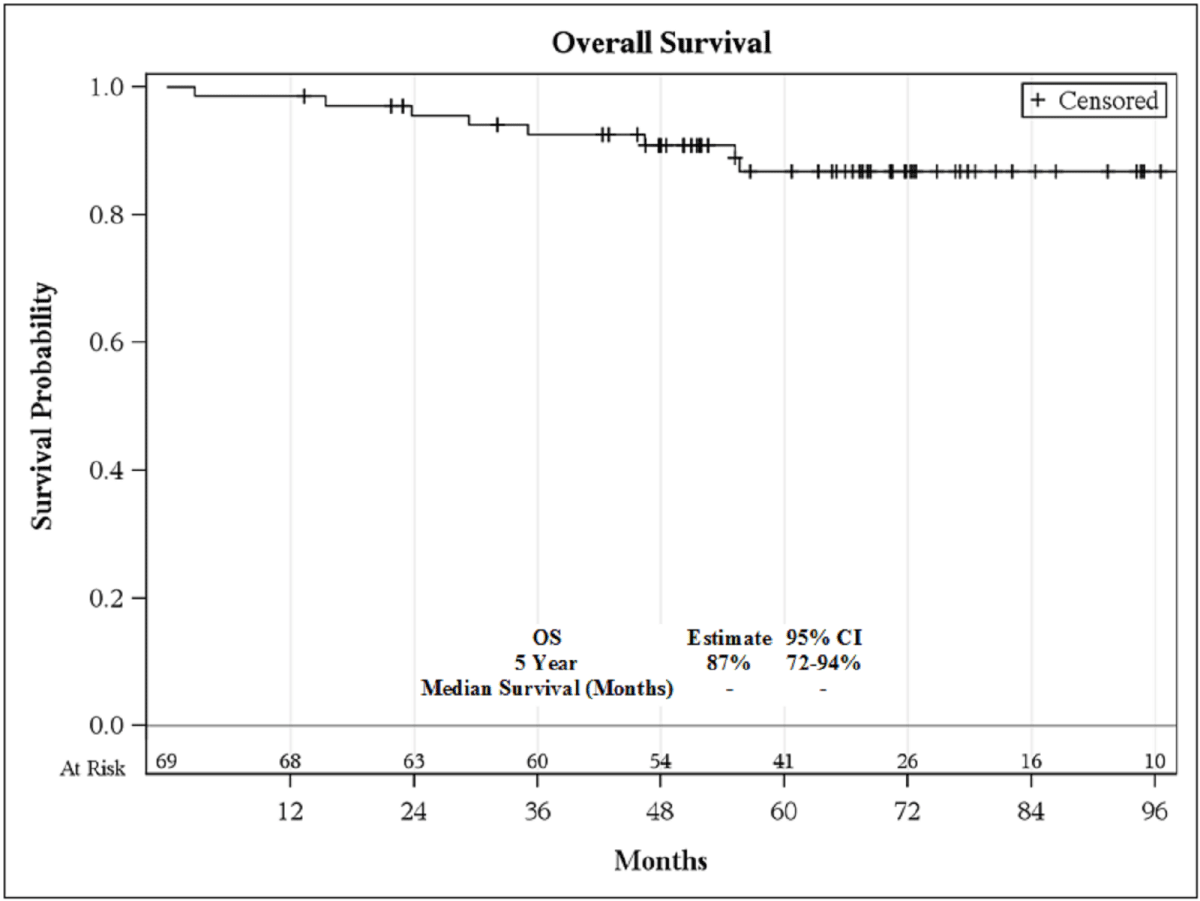
5-year bRFS was 39% (95% C.I., 21-58%; Figure 1), and the 5-year OS
was 87% (72-94%; Figure 2). On univariate analysis, primary Gleason
grade (GG) and overall GS at RP, involved surgical margin(s),
seminal vesicle involvement, nodal involvement, and elevated initial
post-RP PSA (>0.1 ng/mL, within 6 months of RP) were significantly
associated with bRFS (Table 3). Multivariable analysis identified
primary GG at RP (HR=1.80; p<0.01) and post-RP PSA (HR=4.64;
p<0.01) as independently associated with bRFS (Table 3).
In combining these factors, three discrete subsets were identified,
with 5-year estimates of disease control described in Table 4.
Figure 1
Figure 2
Table 3
Table 3
Univariate and Multivariable Analyses of Factor Association with PSA
(Biochemical) Relapse-Free Survival for High-Grade Prostate Cancers at Biopsy,
Managed with Local Therapy Only.
Table 4
Discussion
Gleason 8-10 prostate cancer is characterized by an aggressive
clinical course, including high rates of PSA relapse and poor
disease-specific survival [1-3]. Two major randomized trials have
demonstrated superior disease control and survival outcomes with
the addition of long-course hormone therapy to radiotherapy [5-7],
though such has not been demonstrated for hormone therapy addition
to RP [14,15]. Despite this, several small series have suggested that a
subset of HGPC patients may achieve favorable disease control and
survival with local therapy alone, whether RP or RT (with or without
brachytherapy) [8-12].
Consistent with prior investigations [16-18], the present study
demonstrates high rates of early PSA relapse after primary RP for
HGPC. The 5-year bRFS was only 39%, reflecting both the high
rate of locally invasive features and the elevated risk of lymph node
and distant metastasis. We elected to include patients with HGPC
at biopsy (rather than RP specimen only) in order to consider the
data available at the time of primary intervention decision-making,
at which point either RP or RT are being considered as definitive
local therapy options. The rate of GS concordance between biopsy
and RP specimen was 61% (with 39% downgraded), which aligns with
previous reports specific to high-risk presentation of disease [19,20].
For patients treated with RP, confirmation of Gleason >9 disease
or more advanced pathologic stage (pT3b or pN1) portends worse
outcomes, including biochemical recurrence, metastasis, and death
[9,10]; however, specific subsets of patients with favorable long-term
disease control have been described [25]. Our own findings align
with these data, as primary GG of 4-5 was independently associated
with PSA relapse, yet overall survival remained high (87% at 5 years).
Further, all recurrences initially manifest as PSA relapse, which alone
does not necessarily portend death from prostate cancer. In fact, in
the era of PSA screening and surveillance, over 80% of patients with
PSA failure die of an alternate etiology [3].
Specific to radiotherapy alone, while historical rates of HGPC
control using external-beam RT alone are suboptimal [5-7], patients
enrolled in these trials had more locally advanced tumors and higher
pre-treatment PSA than is seen in the contemporary setting. Recent
evidence suggests impressive PSA control rates employing combined
RT and brachytherapy in selected HGPC cases [11,12]. These
outcomes approach those seen with primary RP (when adjuvant RT
is given in the setting of high-risk features) [16-18].
Detectable initial post-RP PSA (>0.1 ng/mL) was identified as
independently associated with bRFS. Other investigators have noted
this as well [21,22], with worse prognosis associated with earlier
time to PSA failure [22]. While trials of post-operative radiotherapy
improved bRFS irrespective of detectable initial post-op PSA [16,17],
there remains some debate as to whether early salvage radiotherapy
may produce comparable symptom-free and survival outcomes
[23,24].
Given the present study findings, no true “low-risk” group could
be identified, for whom definitive local therapy without systemic
therapy would be appropriate. One major confounding factor was the
low utilization of adjuvant RT in the setting of high-risk pathologic
features, such as involvement of pelvic lymph nodes, seminal vesicles,
extraprostatic tissue, or surgical margin(s). Randomized trials have
demonstrated superior bRFS, [16,17] as well as improved distant
metastasis-free survival benefit [17], for adjuvant RT over RP alone,
reinforcing the importance of local control on systemic involvement.
These differences remained significant for HGPC cases as well [16,17].
Conclusion
High-Gleason grade prostate cancer has a high rate of recurrence after RP alone as demonstrated in the present study by the low rate of freedom from failure after 5 years. However, 5-year OS remains high, suggesting that identification of patients who will recur early versus late is important. Higher primary GG at RP and detectable post-RP PSA (>0.1 ng/mL) are high-risk features that were associated with worse bRFS and warrant investigation along with other prognostic markers to determine the timing of adjuvant therapy for HGPC patients who undergo RP.
References
- Mullins JK, Feng Z, Trock BJ, Epstein JI, Walsh PC, Loeb S. The impact of anatomical radical retropubic prostatectomy on cancer control: the 30-year anniversary. J Urol. 2012; 188: 2219-2224.
- Swanson GP, Hussey MA, Tangen CM, Chin J, Messing E, Canby-Hagino E, et al. Predominant treatment failure in postprostatectomy patients is local: analysis of patterns of treatment failure in SWOG 8794. J Clin Oncol. 2007; 25: 2225-2229.
- Stephenson AJ, Kattan MW, Eastham JA, Bianco FJ, Yossepowitch O, Vickers AJ, et al. Prostate cancer-specific mortality after radical prostatectomy for patients treated in the prostate-specific antigen era. J Clin Oncol. 2009; 27: 4300-4305.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN Guidelines): Prostate Cancer. Version 1. 2015.
- Widmark A, Klepp O, Solberg A, Damber JE, Angelsen A, Fransson P, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. 2009; 373: 301-308.
- Warde P, Mason M, Ding K, Kirkbride P, Brundage M, Cowan R, et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. Lancet 2011; 378: 2104-2111.
- Horwitz EM, Bae K, Hanks GE, Porter A, Grignon DJ, Brereton HD, et al. Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008; 26: 2497-2504.
- Boorjian SA, Karnes RJ, Viterbo R, Rangel LJ, Bergstralh EJ, Horwitz EM, et al. Long-term survival after radical prostatectomy versus external-beam radiotherapy for patients with high-risk prostate cancer. Cancer. 2011; 117: 2883-2891.
- Pierorazio PM, Ross AE, Lin BM, Epstein JI, Han M, Walsh PC, et al. Preoperative characteristics of high-Gleason disease predictive of favourable pathological and clinical outcomes at radical prostatectomy. BJU Int. 2012; 110: 1122-1128.
- Briganti A, Joniau S, Gontero P, Abdollah F, Passoni NM, Tombal B, et al. Identifying the best candidate for radical prostatectomy among patients with high-risk prostate cancer. Eur Urol. 2012; 61: 584-592.
- Taira AV, Merrick GS, Galbreath RW, Butler WM, Lief J, Adamovich E, et al. Distant metastases following permanent interstitial brachytherapy for patients with clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2012; 82: e225-e232.
- Fang LC, Merrick GS, Butler WM, Galbreath RW, Murray BC, Reed JL, et al. High-risk prostate cancer with Gleason score 8-10 and PSA level <15 ng/mL treated with permanent interstitial brachytherapy. Int J Radiat Oncol Biol Phys. 2011; 81: 992-996.
- Gardner JR, Livingston PM, Fraser SF. Effects of exercise on treatment-related adverse effects for patients with prostate cancer receiving androgen-deprivation therapy: a systematic review. J Clin Oncol. 2014; 32: 335-346.
- Spahn M, Briganti A, Capitanio U, Kneitz B, Gontero P, Karnes JR, et al. Outcome predictors of radical prostatectomy followed by adjuvant androgen deprivation in patients with clinical high risk prostate cancer and pT3 surgical margin positive disease. J Urol. 2012; 188: 84-90.
- Shelley MD, Kumar S, Coles B, Wilt T, Staffurth J, Mason MD. Adjuvant hormone therapy for localised and locally advanced prostate carcinoma: a systematic review and meta-analysis of randomised trials. Cancer Treat Rev. 2009; 35: 540-546.
- Van der Kwast TH, Bolla M, Van Poppel H, Van Cangh P, Vekemans K, Da Pozzo L, et al. Identification of patients with prostate cancer who benefit from immediate postoperative radiotherapy: EORTC 22911. J Clin Oncol. 2007; 25: 4178-4186.
- Thompson IM, Tangen CM, Paradelo J, Lucia MS, Miller G, Troyer D, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009; 181: 956-962.
- Watkins JM, Watkins PL, Dufan TA, Koleilat N. High-grade prostate adenocarcinoma (Gleason score >8): survival and disease control following radical prostatectomy versus radiotherapy plus long-course hormone therapy. J Radiat Oncol. 2015; 4: 277-282.
- Rajinikanth A, Manoharan M, Soloway CT, Civantos FJ, Soloway MS. Trends in Gleason score: concordance between biopsy and prostatectomy over 15 years. Urology. 2008; 72: 177-182.
- Truesdale MD, Cheetham PJ, Turk AT, Sartori S, Hruby GW, Dinneen EP, et al. Gleason score concordance on biopsy-confirmed prostate cancer: is pathological re-evaluation necessary prior to radical prostatectomy? BJU Int. 2011; 107: 749-754.
- Shariat SF, Kattan MW, Song W, Bernard D, Gottenger E, Wheeler TM, et al. Early postoperative peripheral blood revers transcription PCR assay for prostate-specific antigen is associated with prostate cancer progression in patients undergoing radical prostatectomy. Cancer Res. 2003; 63: 5874-5878.
- Koo CK, Tuliano P, Komninos C, Choi YD, Chung BH, Hong SJ, et al. Prognostic impact of time to undetectable prostate-specific antigen in patients with positive surgical margins following radical prostatectomy. Ann Surg Oncol. 2015; 22: 693-700.