Research Article
Distinguishing Malignant from Benign Prostate Tumors using Br, Fe, Rb, Sr, and Zn Content in Prostatic Tissue
Rossmann M1, Zaichick S2 and Zaichick V3*
1Department of Biochemistry, University of Cambridge, UK
2Department of Medicine, University of Illinois College of Medicine, USA
3Department of Radionuclide Diagnostics Medical Radiological Research Centre, Russia
*Corresponding author: Zaichick V, Department of Radionuclide
Diagnostics Medical Radiological
Research Centre, Russia
Published: 13 Jul 2016
Cite this article as: Rossmann M, Zaichick S, Zaichick V.
Distinguishing Malignant from Benign
Prostate Tumors using Br, Fe, Rb, Sr,
and Zn Content in Prostatic Tissue. Clin
Oncol. 2016; 1: 1054.
Abstract
Contents of Br, Fe, Rb, Sr, and Zn in normal (n=37), benign hypertrophic (n=43) and cancerous
tissues (n=60) of the human prostate gland were investigated by radionuclide-induced (109Cd)
energy dispersive X-ray fluorescent (EDXRF). Mean values (M±SΕΜ) for mass fraction (mg/kg
on dry mass basis) of trace element in the normal tissue were as follows: Br 40.6±5.6, Fe 118±8,
Rb 16.3±1.1, Sr 2.5±0.4, and Zn 1154±119, respectively. Mean values (M±SΕΜ) for ratio of mass
fractions were: Zn/Br 39.1±6.2, Zn/Fe 11.2±1.3, Zn/Rb 71.7±9.0, and Zn/Sr 534±83, respectively. It
was observed that in benign hypertrophic tissues the levels of Br, Fe, Rb, Sr, and Zn were equal to
those in normal prostate tissues. By contrast, the levels of Rb and Zn were significantly lower and
those of Br and Sr were significantly higher in cancerous tissues than in normal tissues. The Zn mass
fraction, as well as Zn/Br and Zn/Sr ratios, were the most informative indicators for distinguishing
malignant from benign prostate with sensitivity, specificity, and accuracy in the ranges 95-100%,
92-98%, and 95-98%, respectively. Obtained data allowed us to adequately evaluate the importance
of trace element content and their ratios for the diagnosis of prostate cancer.
Keywords: Zinc; Trace elements; Intact prostate; Benign prostatic hyperplasia; Prostate cancer;
Energy-dispersive X-ray fluorescent analysis
Introduction
The prostate gland may be a source of many health problems in past middle age men, the most common being benign prostatic hyperplasia (BPH), and prostatic carcinoma (PCa). BPH is a noncancerous enlargement of the prostate gland leading to obstruction of the urethra and can significantly impair quality of life [1]. The prevalence of histological BPH is found in approximately 50-60% of males age 40-50, in over 70% at 60 years old and in greater than 90% of men over 70 [2,3]. In many Western industrialized countries, including North America, PCa is the most frequently diagnosed form of noncutaneous malignancy in males and, except for lung cancer, is the leading cause of death from cancer [4-9]. Approximately one in eight men will be diagnosed with prostate cancer at some point of their lifetime. Although the etiology of BPH and PCa is unknown, many trace elements have been highlighted in the literature in relation to the development of these prostate diseases [10-29]. Trace elements have essential physiological functions such as maintenance and regulation of cell function and signalling, gene regulation, activation or inhibition of enzymatic reactions, neurotransmission, and regulation of membrane function. Essential or toxic (mutagenic, carcinogenic) properties of trace elements depend on tissue-specific need or tolerance, respectively [30]. Excessive accumulation, deficiency or an imbalance of the trace elements may disturb the cell functions and may result in cellular degeneration, death and malignant transformation [31]. In previous study significant changes of trace element contents in hyperplastic and cancerous prostate in comparison with those in the normal prostatic tissue were observed [32-53]. Moreover, a significant informative value of Zn content as a tumor marker for PCa diagnostics was shown [54]. Hence it is possible that besides Zn, some other trace elements also can be used as tumor markers for distinguish between benign and malignant prostate. Current methods applied for measurement of trace element contents in human tissue samples include a number of techniques. Among these methods the energy dispersive X-ray fluorescence technique (EDXRF) is one of the most simple, fast, reliable, efficient, and available techniques. There are many different kinds of EDXRF devises on the market and technical possibilities of this method improve rapidly. Therefore, the present study had had three aims. The main objective was to assess the Br, Fe, Rb, Sr, and Zn contents as well as Zn/Br, Zn/Fe, Zn/Rb, and Sr/Zn ratios in intact prostate of healthy men aged over 40 years and in the prostate gland of age-matched patients, who had either BPH or PCa using 109Cd-radionuclide induced EDXRF analysis. The second aim was to compare the levels of trace elements in normal, hyperplastic, and cancerous prostate, and the third aim was to evaluate the trace element content for diagnosis of prostate cancer.
Table 1
Table 1
EDXRF data Br, Fe, Rb, Sr, and Zn mass fraction in the IAEA H-4 (animal muscle) reference material compared to certified values (mg/kg, dry mass basis).
Table 2
Table 2
Some statistical parameters of Br, Fe, Rb, Sr, and Zn mass fraction (mg/kg, dry mass basis) and the Zn/Br, Zn/Fe, Zn/Rb, and Zn/Sr mass fraction ratios in
normal, benign hypertrophic and cancerous prostate.
Material and Methods
All patients studied (n=103) were hospitalized in the Urological
Department of the Medical Radiological Research Centre. Transrectal
puncture biopsy of suspicious indurated regions of the prostate
was performed for every patient, to permit morphological study of
prostatic tissue at these sites and to estimate their chemical element
contents. In all cases the diagnosis has been confirmed by clinical
and histopathological assessment of biopsy and surgically resected
materials. A histological examination in the control group was
used to establish the age norm, as well as to confirm the absence of
microadenomatosis and latent cancer.
The age of 43 patients with BPH ranged from 38 to 83 years, the
mean being 66±8 years (M±SD). The 60 patients aged 40-79 suffered
from PCa. Their mean age was 65±10 years. Intact prostates were
removed at necropsy from 37 men (mean age 55±11 years, range
41-79) who had died suddenly. The majority of deaths were due to
trauma.
Tissue samples were divided into two parts. One part of samples
was used for histological and the other for trace element analysis.
For the purpose of the latter samples were weighed, freeze-dried and
then homogenized. The pounded sample weighing about 8 mg was
applied to the piece of Scotch tape that served as an adhesive fixing
backing. To determine the contents of the elements by comparison
with a known standard, aliquots of commercial, chemically pure
compounds were used. The microliter standards were placed on disks
made of thin, ash-free filter papers fixed on the Scotch tape pieces
and dried in a vacuum. Ten subsamples of the Certified Reference
Material (CRM) IAEA H-4 (animal muscle) weighing about 8 mg
were analyzed to estimate the precision and accuracy of results. The
CRM IAEA H-4 subsamples were prepared in the same way as the
samples of dry homogenized prostate. Details of the relevant facility
for EDXRF, source with 109Cd radionuclide, methods of analysis and
the results of quality control were presented in our earlier publications
concerning the EDXRF analysis of human prostate tissue [17,55].
All prostate samples were prepared in duplicate and mean values
of trace element contents were used in final calculation. Using the
Microsoft Office Excel software, the summary of statistics, arithmetic
mean, standard deviation, standard error of mean, minimum and
maximum values, median, percentiles with 0.025 and 0.975 levels was
calculated for trace element contents in normal, benign hyperplastic
and cancerous prostate. The same programs were used to estimate
the inter-correlations of trace element contents. The reliability of
difference in the results between the three groups of prostate samples
was evaluated by Student’s t-test.
The study was approved by the Ethical Committees of the Medical
Radiological Research Centre, Obninsk.
Table 3
Table 3
Comparison of mean values (M±SEM) of Br, Fe, Rb, Sr, and Zn mass fraction (mg/kg, dry mass basis) and the Zn/Br, Zn/Fe, Zn/Rb, and Zn/Sr mass fraction
ratios in normal, benign hypertrophic and cancerous prostate.
Table 4
Table 4
Median, minimum and maximum value of means of Br, Fe, Rb, Sr, and Zn mass fraction in normal, benign hypertrophic and cancerous prostate according to
data from the literature in comparison with our results (mg/kg, dry mass basis).
Results
Table 1 depicts our data for 5 trace elements in ten sub- samples
of CRM IAEA H-4 (animal muscle) and the certified values of this
material.
Table 2 presents certain statistical parameters (arithmetic mean,
standard deviation, standard error of mean, minimal and maximal
values, median, percentiles with 0.025 and 0.975 levels) of the Br,
Fe, Rb, Sr, Zn mass fraction and the Zn/Br, Zn/Fe, Zn/Rb, Zn/Sr
mass fraction ratios in normal, benign hypertrophic and cancerous
prostate. The ratios of means and the reliability of difference between
mean values of Br, Fe, Rb, Sr, Zn mass fractions and the Zn/Br, Zn/
Fe, Zn/Rb, Zn/Sr mass fraction ratios in normal, benign hypertrophic
and cancerous prostate are presented in Table 3.
The comparison of our results with published data for Br, Fe,
Rb, Sr, and Zn mass fraction in normal, benign hypertrophic and
cancerous prostate is shown in Table 4.
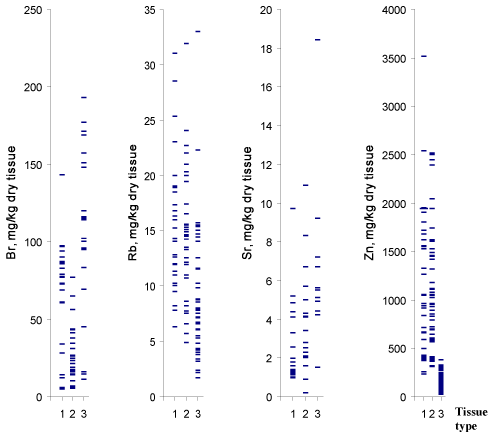
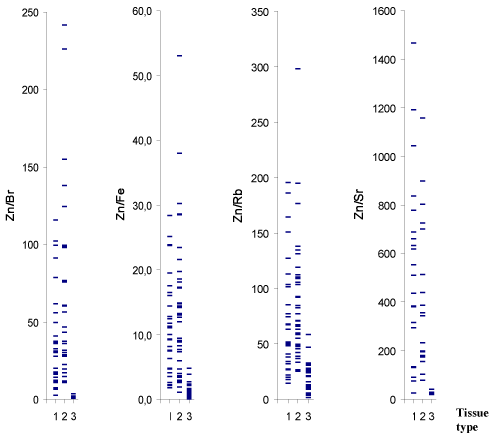
Figure 1 and 2 depict individual data sets for Br, Fe, Rb, Sr, Zn
mass fraction and for the Zn/Br, Zn/Fe, Zn/Rb, Zn/Sr mass fraction
ratios, respectively, in all samples of normal, benign hypertrophic and
cancerous prostate.
Discussion
As was shown by us [17,55] the use of CRM IAEA H-4 as a CRM
for the analysis of samples of prostate tissue can be seen as quite
acceptable. Good agreement of the Br, Fe, Rb, Sr, and Zn contents
analyzed by EDXRF with the certified data of CRM IAEA H-4. Table
1 indicates an acceptable accuracy of the results obtained in the study
of trace elements of the prostate presented in Tables 2-4. The mean
values and all selected statistical parameters were calculated for 5
trace elements (Br, Fe, Rb, Sr, and Zn) and for 4 ratios (Zn/Br, Zn/
Fe, Zn/ Rb, and Zn/Sr) of chemical element mass fractions (Table 2).
The mass fraction of Br, Fe, Rb, Sr, and Zn were measured in all, or
a major portion of normal, BPH and PCa samples. From Table 3, it
is observed that in benign hypertrophic tissues the mass fractions of
Br, Fe, Rb, Sr, and Zn not differ from normal levels. In cancerous
tissue the mass fractions of Rb (p<0.01) and Zn (p<0.001) are lower,
and mass fractions of Br (p<0.01) and Sr (p<0.01) are significantly
higher than in normal tissues of the prostate. Except for Fe and Sr, the
mass fractions of all the other elements show significant variations in
cancerous tissues when compared with benign hypertrophic tissues
of the prostate. The mass fractions of Rb (p<0.01) and Zn (p<0.001)
are lower and mass fraction of Br (p<0.01) is higher than in benign
hypertrophic tissues. The mean level of Zn/Br mass fraction ratio
in BPH is significantly (p<0.05) higher than in normal prostate. In
cancerous tissue the Zn/Br, Zn/Fe, Zn/ Rb, and Zn/Sr mass fraction
ratios are significantly (p<0.001) lower than in normal and benign
hypertrophic tissues. The results for all trace element contents in the
prostates of the control group (mean age 55±11 years, range 41-79)
are in accordance with our earlier findings in prostates of apparently
healthy men aged 41-60 [17]. Values obtained for Br, Fe, Rb, Sr, and
Zn contents (Table 4) agree well with median of mean values cited by
other researches for the human prostate [32-44]. Data of the literature
also includes samples obtained from patients who died from different
diseases. A number of values for chemical element mass fractions
were not expressed on a dry mass basis in the cited literature.
Therefore, we calculated these values using published data for water -
80% [43] and ash - 1% on wet mass basis [56] contents in the prostate
of adult men. Our results for Br, Fe, and Zn are in accordance with
the medians of earlier findings in benign hypertrophic tissues of
prostate (Table 4). No published data referring Rb and Sr contents in
benign hypertrophic tissues of the prostate were found. In cancerous
prostate tissues our results were comparable with published data for
Fe and Zn contents and two orders of magnitude higher for Br (Table
4). No published data referring Rb and Sr contents of cancerous
prostate tissue were found. In BPH the mean Br, Fe, Rb, Sr, and Zn
mass fractions were equal to normal prostate (Table 3). This result
is in accordance with earlier findings for Fe [34,57,58] and for Zn
[34,35,39,43,57-69]. In cancerous tissues mean values for Br and Sr
mass fractions were significantly higher whereas mean values for Rb
and Zn mass fractions were significantly lower than in normal prostate
(Table 3). Data obtained for Zn are in agreement with previously
reported findings for this element [35,39,40,57-61,63-65,67-71]. In
cancerous tissues mean values for Br, Fe, and Sr mass fractions were
equal to or greater than those for benign hypertrophic prostate tissues
and Rb and Zn mass fractions were significantly lower (Table 3). This
result is in accordance with earlier findings for Fe [35,41,58,72] and
for Zn [35,39,41,43,44,57,60,61,63-69,71,73-78]. No published data
referring to mass fraction ratios of Br, Fe, Rb, Sr, and Zn in normal,
benign hypertrophic and cancerous prostate could be found.
Characteristically, elevated or deficient levels of trace elements
observed in cancerous tissues are discussed in terms of their potential
role in the initiation, promotion, or inhibition of prostate cancer. In
our opinion, abnormal levels of many trace elements in cancerous
tissue could be the consequence of malignant transformation.
Compared to other soft tissues, the human prostate has higher levels
of Zn, Ca, Mg and some other trace elements [16,17,79-83]. These
data suggests that these elements could be involved in functional
features of prostate tissue [84-88]. Malignant transformation is
accompanied by a loss of tissue-specific functional features, which
leads to a significant reduction in the contents of elements associated
with functional characteristics of the human prostate tissue (Zn and,
probably, Rb – (Table 3). Therefore, it is plausible that the reason
for the emergence and development of cancer is associated with
abnormally high concentration of some metals in the prostate tissue
of older men [16,17,20-24]. The same reasoning could be applied to
benign hypertrophic tissue (it retains functional features of normal
prostate tissue) and its increased levels Sr and Zn (Table 3). Trace
elemental analysis of prostate tissue could become a powerful
diagnostic tool. To a large extent, the resumption of the search for
new methods for early diagnosis of PCa was due to experience gained
in a critical assessment of the limited capacity of the serum prostate
specific antigen (PSA) test [89]. In addition to the PSA serum test
and morphological study of needle-biopsy cores of the prostate,
the development of other highly precise testing methods seems to
be very useful. Experimental conditions of the present study were
approximated to the hospital conditions as closely as possible. In all
cases we analyzed a part of the material obtained from a transrectal
puncture biopsy of the indurated site in the prostate. Therefore, our
data allow us to evaluate adequately the importance of trace element
content information for the diagnosis of PCa.
Tissue content of Br, Rb, and Zn are significantly different in most
cancerous tissues as compared to normal and benign hypertrophic
tissues (Table 3). As is evident from individual data sets (Figure 1),
the Zn mass fraction is one of the most informative for a differential
diagnosis. If 350 mg/kg dry tissue (M±2.5SD) is the value of Zn mass
fraction assumed to be the upper limit for PCa (Figure 1) and an
estimation is made for “PCa or intact and BPH tissue”, the following
values are obtained:
Sensitivity = {True Positives (TP) / [TP + False Negatives (FN)]}
·100% = 98±2%;
Specificity = {True Negatives (TN) / [TN + False Positives (FP)]}
·100% = 92±3%;
Accuracy = [(TP+TN) / (TP+FP+TN+FN)] ·100% = 95±2%.
The number of people (samples) examined was taken into
account for calculation of confidence intervals [90]. In other words,
if Zn contents in a prostate biopsy sample do not exceed 350 mg/kg
dry tissue, one could diagnose a malignant tumor with an accuracy of
95±2%. Thus, using the Zn-test makes it possible to diagnose cancer
in 98±2% cases (sensitivity). We have previously shown that not only
the absolute values of the chemical elements can be used successfully
for diagnostic purposes but also their ratios and other mathematic
combinations [91]. The use of the relations between mass fractions of
chemical elements is particularly promising for the development of in
vivo diagnostic methods, including the diagnosis of PCa. As is evident
from individual data sets (Figure 2), the Zn/Br and Zn/Sr mass fraction
ratios are the most informative indicators for a differential diagnosis.
If 2.9 (M±2.5SD) and 40 (M±2.5SD) are the values of Zn/Br and Zn/
Sr mass fraction ratios assumed to be the upper limit for PCa (Figure
2) and an estimation is made for “PCa or intact and BPH tissue”, the
following values are obtained: using Zn/Br ratio - sensitivity 95±5%,
specificity 98±2%, and accuracy 97±2%; using Zn/Sr ratio - sensitivity
100-10%, specificity 982%, and accuracy 98±2%. It should be noted,
however, that Br is a component of many tranquilizers. It is possible
that the increase in Br content could be explained by uncontrolled use
of tranquilizers in the group of PCa patients. Therefore, for diagnostic
purposes, data for Br content should be used with caution. Possibility
of using the Zn/Sr ratio is limited by difficulties in determining the Sr
content by EDXRF because the content of this element in the prostate
tissue is close to the limit of detection.
Figure 1
Figure 1
Individual data sets for Br, Rb, Sr, and Zn mass fractions in samples
of normal (1), benign hypertrophic (2) and cancerous prostate (3).
Conclusion
In this work, trace elemental analysis was carried out in the tissue samples of normal, benign hypertrophic, and carcinomatous prostates using EDXRF. It was shown that EDXRF is an adequate analytical tool for the non-destructive determination of Br, Fe, Rb, Sr, and Zn content in the tissue samples of human prostate, including needle-biopsy cores. It was observed that in benign hypertrophic tissues the contents of Br, Fe, Rb, Sr, and Zn were equal to those in normal prostate tissues. It is possible that elevated levels of Sr and Zn initiate and promote prostate cancer by oxidative DNA damage, which is caused by an increase in generation of free radicals and a decrease in the antioxidant defense capacity of cells. The contents of Rb and Zn were significantly lower and those of Br and Sr were significantly higher in cancerous tissues than in normal tissues. In our opinion, the abnormal decrease in levels Rb and Zn in cancerous tissue could be a consequence of malignant transformation. Finally, we propose to use the Zn mass fraction in a needle-biopsy core as an accurate tool to diagnose prostate cancer. Further studies on larger number of samples are required to confirm our findings, to study the impact of the trace elements on prostate cancer etiology and to examine the long-term pathological outcome.
Figure 2
Figure 2
Individual data sets for Zn/Br, Zn/Fe, Zn/Rb, and Zn/Sr mass
fraction ratios in samples of normal (1), benign hypertrophic (2) and
cancerous prostate (3).
Acknowledgements
We are grateful to Dr. Tatyana Sviridova, Medical Radiological Research Center, Obninsk, and to the late Prof. A.A. Zhavoronkov, Institute of Human Morphology, Russian Academy of Medical Sciences, Moscow, for supplying prostate samples.
References
- Kirby RS. The natural history of benign prostatic hyperplasia: what have we learned in the last decade? Urology. 2000; 56: 3-6.
- Roehrborn C, McConnell J. Etiology, pathophysiology, epidemiology and natural history of benign prostatic hyperplasia. In: Walsh P, Retik A, Vaughan E, Wein A, editors. Campbell’s Urology. 8th ed. Philadelphia: Saunders. 2002; 1297-1336.
- Lepor H. Pathophysiology of benign prostatic hyperplasia in the aging male population. Rev Urol. 2005; 7: S3-S12.
- Oliver SE, Gunnell D, Donovan JL. Comparison of trends in prostatecancer mortality in England and Wales and the USA. Lancet. 2000; 355: 1788-1789.
- Kumar RJ, Barqawi AB, Crawford ED. Epidemiology of prostate cancer. Business Briefing: US Oncology Review. 2004; 1-6.
- Maddams J, Brewster D, Gavin A, Steward J, Elliott J, Utley M, et al. Cancer prevalence in the United Kingdom: estimates for 2008. Br J Cancer. 2009; 101: 541-547.
- Lutz JM, Francisci S, Mugno E, Usel M, Pompe-Kirn V, Coebergh JW, et al. Cancer prevalence in Central Europe: the EUROPREVAL Study. Ann Oncol. 2003; 14: 313-322.
- Möller T, Anderson H, Aareleid T, Hakulinen T, Storm H, Tryggvadottir L, et al. Cancer prevalence in Northern Europe: the EUROPREVAL study. Ann Oncol. 2003; 14: 946-957.
- De Angelis R, Grande E, Inghelmann R, Francisci S, Micheli A, Baili P, et al. Cancer prevalence estimates in Italy from 1970 to 2010. Tumori. 2007; 93: 392-397.
- Waalkes MP, Rehm S. Cadmium and prostate cancer. J Toxicol Environ Health. 1994; 43: 251-269.
- Zaichick V, Zaichick S. Role of zinc in prostate cancerogenesis. In: Anke M, et al., editors. Mengen und Spurenelemente. 19. Arbeitstagung. Jena: Friedrich-Schiller-Universitat; 1999, p.104-115.
- Platz EA, Helzlsouer KJ. Selenium, zinc, and prostate cancer. Epidemiol Rev. 2001; 23: 93-101.
- Zaichick V. INAA and EDXRF applications in the age dynamics assessment of Zn content and distribution in the normal human prostate. J Radioanal Nucl Chem. 2004; 262: 229-234.
- Gray MA, Centeno JA, Slaney DP, Ejnik JW, Todorov T, Nacey JN. Environmental exposure to trace elements and prostate cancer in three New Zealand ethnic groups. Int J Environ Res Public Health. 2005; 2: 374-384.
- Zaichick S, Zaichick V. INAA application in the age dynamics assessment of Br, Ca, Cl, K, Mg, Mn, and Na content in the normal human prostate. J Radioanal Nucl Chem. 2011; 288: 197-202.
- Zaichick S, Zaichick V. The effect of age on Ag, Co, Cr, Fe, Hg, Sb, Sc, Se, and Zn contents in intact human prostate investigated by neutron activation analysis. Appl Radiat Isot. 2011; 69: 827-833.
- Zaichick S, Zaichick V. The Br, Fe, Rb, Sr, and Zn content and interrelation in intact and morphologic normal prostate tissue of adult men investigated by energy dispersive X-ray fluorescent analysis. X-Ray Spectrom. 2011; 40: 464-469.
- Zaichick V, Nosenko S, Moskvina I. The effect of age on 12 chemical element contents in intact prostate of adult men investigated by inductively coupled plasma atomic emission spectrometry. Biol Trace Elem Res. 2012; 147: 49-58.
- Zaichick S, Zaichick V, Nosenko S, Moskvina I. Mass Fractions of 52 Trace Elements and Zinc Trace Element Content Ratios in Intact Human Prostates Investigated by Inductively Coupled Plasma Mass Spectrometry. Biol Trace Elem Res. 2012; 149: 171-183.
- Zaichick V, Zaichick S. Age-related histological and zinc content changes in adult nonhyperplastic prostate glands. Age. 2014; 36: 167-181.
- Zaichick V, Zaichick S. INAA application in the assessment of chemical element mass fractions in adult and geriatric prostate glands. Appl Radiat Isot. 2014; 90: 62-73.
- Zaichick V, Zaichick S. Determination of trace elements in adults and geriatric prostate combining neutron activation with inductively coupled plasma atomic emission spectrometry. Open Journal of Biochemistry. 2014; 1: 16-33.
- Zaichick V, Zaichick S. Use of INAA and ICP-MS for the assessment of trace element mass fractions in adult and geriatric prostate. J Radioanal Nucl Chem. 2014; 301: 383-397.
- Zaichick V. The variation with age of 67 macro- and microelement contents in nonhyperplastic prostate glands of adult and elderly males investigated by nuclear analytical and related methods. Biol Trace Elem Res. 2015; 168: 44-60.
- Zaichick V, Zaichick S. Dietary intake of minerals and prostate cancer: insights into problem based on the chemical element contents in the prostate gland. J Aging Res Clin Practice 2015; 4: 164-171.
- Zaichick V, Zaichick S. Global contamination from uranium: insights into problem based on the uranium content in the human prostate gland. J Environ Health Sci 2015; 1: 1-5.
- Zaichick V, Zaichick S. Variations in concentration and distribution of several androgen-dependent and -independent trace elements in nonhyperplastic prostate gland tissue throughout adulthood. J Androl Gynaecol. 2016; 4: 1-10.
- Zaichick V, Zaichick S. Age-related Changes in Concentration and Histological Distribution of Br, Ca, Cl, K, Mg, Mn, and Na in Nonhyperplastic Prostate of Adults. European Journal of Biology and Medical Science Research. 2016; 4: 31-48.
- Zaichick V., Zaichick S. Variations in concentration and histological distribution of Ag, Co, Cr, Fe, Hg, Rb, Sb, Sc, Se, and Zn in nonhyperplastic prostate gland throughout adulthood. Jacobs Journal of Cell and Molecular Biology. 2016; 011: 1-16.
- Zaichick V. Medical elementology as a new scientific discipline. J Radioanal Nucl Chem. 2006; 269: 303-309.
- Schwartz MK. Role of trace elements in cancer. Cancer Res. 1975; 35: 3481- 3487.
- Kubo H, Hashimoto S, Ishibashi A, Chiba R, Yokota H. Simultaneous determinations of Fe, Cu, Zn, and Br concentrations in human tissue sections. Medical Physics. 1976; 3: 204-209.
- Forssen A. Inorganic elements in the human body. I. Occurrence of Ba, Br, Ca, Cd, Cs, Cu, K, Mn, Ni, Sn, Sr, Y and Zn in the human body. Ann Med Exp Biol. 1972; 50: 99-162.
- Sangen H. The influence of the trace metals upon the aconitase activity in human prostate glands. Jap J Urol. 1967; 58: 1146-1159.
- Jafa A, Mahendra NM, Chowdhury AR, Kamboj VP. Trace elements in prostatic tissue and plasma in prostatic diseases of man. Indian J Cancer. 1980; 17: 34-37.
- Stitch SR. Trace elements in human tissue. I. A semi-quantitative spectrographic survey. Biochem J. 1957; 67: 97-103.
- Soman SD, Joseph KT, Raut SJ, Mulay GD, Parameswaran M, Pandey VK. Studies of major and trace element content in human tissues. Health Phys. 1970; 19: 641-656.
- Tipton IH, Cook MJ. Trace elements in human tissue. Part II. Adult subjects from the United States. Health Phys. 1963; 9: 103-145.
- Brys M, Nawrocka AD, Miekos E, Zydek C, Foksinski M, Berecki A, et al. Zinc and cadmium analysis in human prostate neoplasmas. Biol Trace Element Res, 1997; 59: 145-152.
- Lindholmer C, Glaumann H. Zinc and magnesium in human male reproductive tract. Andrologie. 1972; 4: 231-237.
- Naga Raju GJ, Padala S, Gummuluri AVRM, Muktineni RK, Byreddy SR, Sreerama L, et al. Trace elemental analysis of normal, benign, hypertrophic and cancerous tissues of the prostate gland using the particle-induced X-ray emission technique. Eur J Cancer Prev. 2007; 16: 108-115.
- Kiziler AR, Aydemir B, Guzel S, Alici B, Ataus S, Tuna MB, et al. May the level and ratio changes of trace elements be utilized in identification of disease progression and grade in prostatic cancer? Trace Elements and Electrolytes. 2010; 27: 65-72.
- Györkey F, Min K-W, Huff JA, Györkey P. Zinc and magnesium in human prostate gland: Normal, hyperplastic, and neoplastic. Cancer Res. 1967; 27: 1349-1353.
- Kwiatek WM, Hanson AL, Paluszkiewicz C, Gałka M, Gajda M, Cichocki T. Application of SRIXE and XANES to the determination of the oxidation state of iron in prostate tissue sections. Journal of Alloys and Compounds. 2004; 362: 83-87.
- Zaichick V, Zaichick S. Differences and relationships between morphometric parameters and zinc content in nonhyperplastic and hyperplastic prostate glands. British Journal of Medicine and Medical Research. 2015; 8: 692-706.
- Zaichick V, Zaichick S. Trace element contents in adenocarcinoma of human prostate investigated by energy dispersive X-ray fluorescent analysis. Journal of Adenocarcinoma, 2016; 1: 1-7.
- Zaichick V, Zaichick S. The Bromine, Calcium, Potassium, Magnesium, Manganese, and Sodium Contents in Adenocarcinoma of Human Prostate Gland. J Hematology and Oncology Research. 2016; 2: 1-12.
- Zaichick V, Zaichick S. Trace element contents in adenocarcinoma of the human prostate gland investigated by neutron activation analysis. Cancer Research and Oncology. 2016; 1: 1-10.
- Zaichick V, Zaichick S. Prostatic tissue levels of 43 trace elements in patients with prostate adenocarcinoma. Cancer and Clinical Oncology. 2016; 5: 79-94.
- Zaichick V, Zaichick S. Chemical elemental content / Calcium ratios in tissues of human hyperplastic prostate gland. Journal of Applied Life Sciences International. 2016; 4: 1-11.
- Zaichick V, Zaichick S. Neutron activation analysis of Ag, Co, Cr, Fe, Hg, Rb, Sb, Sc, Se, and Zn contents in benign prostatic hypertrophic tissue. In: Neutron Spectroscopy, Nuclear Structure, Related Topics. Dubna, Moscow Region, Russia: Joint Institute for Nuclear Research; 2016; 437- 442.
- Zaichick V, Zaichick S. Prostatic tissue level of some major and trace elements in patients with BPH. Jacobs Journal of Nephrology and Urology. 2016; 3: 1-10.
- Zaichick V, Zaichick S. Levels of 43 Trace Elements in Hyperplastic Prostate Tissues. British Journal of Medicine and Medical Research. 2016; 15: 1-12.
- Zaichick V, Sviridova T, Zaichick S. Zinc in human prostate gland: normal, hyperplastic and cancerous. Int Urol Nephrol. 1997; 29: 565-574.
- Zaichick S, Zaichick V. Method and portable facility for energy-dispersive X-ray fluorescent analysis of zinc content in needle-biopsy specimens of prostate. X-Ray Spectrom. 2010; 39: 83-89.
- Saltzman BE, Gross SB, Yeager DW, Meiners BG, Gartside PS. Total body burdens and tissue concentrations of lead, cadmium, copper, zinc, and ash in 55 human cadavers. Environ Res. 1990; 52: 126-145.
- Hienzsc E. Schneider H-J, Anke M. Vergleichende Untersuchungen zum Mengen- und Spurenelementgehalt der normalen Prostata, des Prostataadenoms und des Prostatakarzinoms. Zeitschrift für Urologie und Nephrologie. 1970; 63: 543-546.
- Picurelli L, Olcina PV, Roig MD, Ferrer J. Determination of Fe, Mg, Cu, and Zn in normal and pathological prostatic tissue. Actas Urol Esp. 1991; 15: 344-350.
- Dhar NK, Goel TC, Dube PC, Chowdhury AR, Kar AB. Distribution and concentration of zinc in the subcellular fractions of benign hyperplastic and malignant neoplastic human prostate. Exp Mol Pathol. 1973; 19: 139- 142.
- Habib, FK, Hammond GL, Lee JR, Dawson JB, Mason MK, Stitch SR, et al. Metal-androgen interrelationships in carcinoma and hyperplasia of the human prostate. J Endocrinol. 1976; 71: 133-142.
- Schrodt GR, Hall T, Whitmore WRJr. The concentration of zinc in diseased human prostate glands. Cancer.1964; 17: 1555-1566.
- Leissner KM, Fielkegard B, Tisell LE. Concentration and content of zinc in human prostate. Invest Urol. 1980; 18: 32-35.
- Maganto PE. Zinc levels in the normal and pathological prostate tissue. Actas Urol Esp. 1980; 4: 15-20.
- Lahtonen R. Zinc and cadmium concentrations in whole tissue and in separated epithelium and stroma from human benign prostatic hypertrophic glands. The Prostate. 1985; 6: 177-183.
- Wennrich R, Feustel A. Determination of cadmium and zinc in human prostatic tissues by flameless AAS. Z Med Lab Diagn. 1985; 26: 365-369.
- Feustel A, Wennrich R, Dittrich H. Zinc, cadmium and selenium concentrations in separated epithelium and stroma from prostatic tissues of different histology. Urol Res. 1987; 15: 161-163.
- Ogunlewe JO, Osegbe DN. Zinc and cadmium concentrations in indigenous blacks with normal, hypertrophic, and malignant prostate. Cancer. 1989; 63: 1388-1392.
- Borowiec D, Spruch T, Juszkiewicz M. The measurements of the selected trace element levels in the prostate. Urologia Polska. 1995; 48: 1-2.
- Sapota A, Daragó A, Taczalski J, Kilanowicz A. Disturbed homeostasis of zinc and other essential elements in the prostate gland dependent on the character of pathological lesions. Biometals. 2009; 22: 1041-1049.
- Müntzing J, Nilson T, Polasek J. Zinc and β-glucuronidase in human prostate. Scand J Urol Nephrol (Stockholm). 1974; 8: 87-90.
- Marezynska A, Kulpa J, Lenko J. The concentration of zinc in relation to fundamental elements in the diseases human prostate. Int Urol Nephrol. 1983; 15: 257-265.
- Yaman M, Atici D, Bakirdere S, Akdeniz I. Comparison of trace metal concentrations in malignant and benign human prostate. J Med Chem. 2005; 48: 630-634.
- Mawson CA, Fischer MJ. The occurrence of zinc in the human prostate gland. Canadian Journal of Medical Sciences. 1952; 30: 336-339.
- Hoare R, Delory GE, Penner DW. Zinc and acid phosphatase in the human prostate. Cancer. 1956; 9: 721-726.
- Shirakawa T. Clinical and experimental study on quantitative analysis of zinc in prostate. Acta Urol Japon. 1961; 7: 352-362.
- Wallace AM, Grant JK. Effect of zinc on androgen metabolism in the human hyperplastic prostate. Biochem Soc Trans. 1975; 3: 540-542.
- Habib FK. Evaluation of androgen metabolism studies in human prostate cancer – correlation with zinc levels. Prev Med. 1980; 9: 650-656.
- Vartsky D, Shilstein S, Bercovich A, Huszar M, Breskin A, Chechik R, et al. Prostatic zinc and prostate specific antigen: an experimental evaluation of their combined diagnostic value. J Urol. 2003; 170: 2258-2262.
- Zaichick S, Zaichick V. Relations of morphometric parameters to zinc content in paediatric and nonhyperplastic young adult prostate glands. Andrology. 2013; 1: 139–146.
- Zaichick V, Zaichick S. The effect of age on Br, Ca, Cl, K, Mg, Mn, and Na mass fraction in pediatric and young adult prostate glands investigated by neutron activation analysis. Appl Radiat Isot, 2013; 82: 145-151.
- Zaichick V, Zaichick S. INAA application in the assessment of Ag, Co, Cr, Fe, Hg, Rb, Sb, Sc, Se, and Zn mass fraction in pediatric and young adult prostate glands. J Radioanal Nucl Chem. 2013; 298: 1559-1566.
- Zaichick V, Zaichick S. NAA-SLR and ICP-AES Application in the Assessment of Mass Fraction of 19 Chemical Elements in Pediatric and Young Adult Prostate Glands. Biol Trace Elem Res. 2013; 156: 357-366.
- Zaichick V, Zaichick S. Use of Neutron Activation Analysis and Inductively Coupled Plasma Mass Spectrometry for the Determination of Trace Elements in Pediatric and Young Adult Prostate. American Journal of Analytical Chemistry. 2013; 4: 696-706.
- Zaichick V, Zaichick S. Androgen-dependent chemical elements of prostate gland. Androl Gynecol: Curr Res. 2014; 2:2.
- Zaichick V, Zaichick S. Relations of Bromine, Iron, Rubidium, Strontium, and Zinc Content to Morphometric Parameters in Pediatric and Nonhyperplastic Young Adult Prostate Glands. Biol Trace Elem Res. 2014; 157: 195-204.
- Zaichick V, Zaichick S. Relations of the neutron activation analysis data to morphometric parameters in pediatric and nonhyperplastic young adult prostate glands. Advances in Biomedical Science and Engineering. 2014; 1: 26-42.
- Zaichick V, Zaichick S. Relations of the Al, B, Ba, Br, Ca, Cl, Cu, Fe, K, Li, Mg, Mn, Na, P, S, Si, Sr, and Zn mass fractions to morphometric parameters in pediatric and nonhyperplastic young adult prostate glands. BioMetals. 2014; 27: 333-348.
- Zaichick V, Zaichick S. The Distribution of 54 Trace Elements Including Zinc in Pediatric and Nonhyperplastic Young Adult Prostate Gland Tissues. Journal of Clinical and Laboratory Investigation Updates. 2014; 2: 1-15.
- Catalona WJ. Clinical utility of measurements of free and total prostatespecific antigen (PSA): A review. The Prostate. 1996; 7: 64–69.
- Genes VS. Simple methods for cybernetic data treatment of diagnostic and physiological studies. Moscow: Nauka; 1967.
- Zaichick V, Dunchik VN, Zherbin EA, Leonov AI, Sviridova TV. Method for differential diagnostics of prostate malignant and benign tumours. Certificate of invention, Russia, 1977.