Case Report
A Case of High Grade Advanced Neuroendocrine Cancer of the Bladder Treated with Bevacizumab, Cisplatin, Etoposide and Cyclophosphamide-Based Chemotherapy
Sroussi M1, Fournier L2, Blons H3 and Oudard S1*
1Department of Oncology, European Georges Pompidou Hospital, René Descartes University, France
2Department of Radiology, European Georges Pompidou Hospital, René Descartes University, France
3Department of Biochemistry, European Georges Pompidou Hospital, René Descartes University, France
*Corresponding author: Stéphane Oudard, Department of Oncology, European Georges Pompidou Hospital, René Descartes University, 20 rue Leblanc, 75015 Paris, France
Published: 10 Jun, 2016
Cite this article as: Sroussi M, Fournier L, Blons H, Oudard
S. A Case of High Grade Advanced
Neuroendocrine Cancer of the Bladder
Treated with Bevacizumab, Cisplatin,
Etoposide and Cyclophosphamide-
Based Chemotherapy. Clin Oncol.
2016; 1: 1050.
Abstract
Neuroendocrine bladder cancer accounts for <1% of all bladder cancers. There is no standard
treatment for neuroendocrine bladder cancer; instead therapy is mainly based on agents used
in small cell lung cancer. The case report details a patient with neuroendocrine bladder cancer
who had disease progression after conventional chemotherapy based on etoposide and cisplatin.
During second-line chemotherapy with etoposide, cisplatin and ifosfamide the patient experienced
a high-grade neurological adverse event, without any decrease in tumor burden. Third-line
chemotherapy with cisplatin, cyclophosphamide, etoposide and bevacizumab was associated with
an almost complete response but the patient died of hematological toxicity. The good response to
the combination of cisplatin, cyclophosphamide, etoposide and bevacizumab suggests that antiangiogenic
therapies may be a treatment option for neuroendocrine bladder cancer that deserved
further study.
Keywords: Small cell bladder cancer; Bevacizumab; Cisplatin; Etoposide; Cyclophosphamide;
Hematotoxicity
Introduction
Neuroendocrine bladder cancer is a very rare type of bladder cancer. As a result, there are no large prospective clinical trials investigating treatment options for this type of cancer. Instead, treatment is often based on that used in small cell lung cancer, although surgical options differ between the two cancer types [1,2]. Neuroendocrine bladder tumors show significant heterogeneity, particularly with respect to sequencing, and their pathogenesis is not well defined. The primary theory is that a multipotent common stem cell has the ability to differentiate into various cell types depending on the influence of specific expression of progression-related genes. The neuroendocrine component is frequently associated with transitional cell carcinoma within the same tumor [1,3].
Case Report
A 56-year-old woman presented with high-grade large and small cell neuroendocrine bladder cancer. Her medical history was unremarkable apart from active smoking (about 40 pack-years). Her first symptom of bladder cancer was hematuria. Cystoscopy detected a lesion on the right wall face of the bladder and the patient underwent a transurethral resection. One month later, she had loco-regional recurrence. Radiological evaluation showed a tumor located on the right wall of the bladder, which measured 63x47mm (maximum standard uptake value [SUV max]: 12.2) before initial chemotherapy; neuroendocrine biomarkers were positive. The patient was treated with preoperative chemotherapy using cisplatin and etoposide, with granulocyte colony-stimulating factor (G-CSF) support. After 2 cycles, radiological evaluation detected progressive disease (Figure 1). The tumor had increased in size to 86x59 mm (SUV max: 12.3). Chemotherapy was stopped and the patient underwent cystectomy with lymph node dissection. Histological analysis showed complete resection, but there was lymph node invasion; the tumor was ypT3N2. Next Generation Sequencing was performed, which showed 20% tumor cells. More than 500 hotspot mutation regions of 22 genes associated with lung tumor tissue were studied with the AmpliSeq Colon and Lung Cancer Panel V2 (Life Technologies). No mutations in KRAS, EGFR, BRAF, NRAS known to have therapeutic or prognostic impact were detected. Two abnormalities were identified, the predictive value of which was unknown. There was a gain-of-function mutation of TP53 (p.E285K, c.853G>A, allelic ratio 75%) that is often associated with poor prognosis [4], and a nonsense mutation of SMAD4 (p.GIn245*, c.733C>T, allelic ratio 29%) meaning that the TGF-beta pathway was impaired. One month later, the patient had an aggressive locoregional recurrence. The patient had severe abdominal pain, treated with opioids. There were several tumor nodules in the pelvis, one of 95mm (SUV max: 19) and another of 77mm (SUV max: 18.3) (Figure 2). Mesenteric lymphadenopathy measuring 16mm and 9mm was also detected. Second-line chemotherapy with cisplatin (20mg/m2/ day for 5 days), etoposide (75mg/m2/day for 5 days) and ifosfamide (1200mg/m2/day for 5 days) with G-CSF was given. Unfortunately, the patient developed ifosfamide-induced encephalopathy at the start of treatment with this regimen. Magnetic resonance imaging (MRI) did not find any brain metastasis. Neurological symptoms resolved after treatment with methylene blue, thiamine, and albumin. However, inclusion of chemotherapy including ifosfamide was deemed too risky, especially as the disease remained progressive on radiological evaluation (+18%): 106mm (vs. 95mm), 85 mm (vs. 77mm), 23mm (vs. 16mm) and 18mm (vs. 9mm). A decision was made to initiate third-line chemotherapy with cisplatin (20mg/m2/day for 5 days), etoposide (75mg/m2/day for 5 days), cyclophosphamide (400mg/m2/ day for 3 days) and bevacizumab (15mg/kg on day 1), plus G-CSF. There was evidence of a partial response on radiological evaluation: 96mm (vs. 106mm), 84mm (vs. 85mm), 17mm (vs. 23mm) and 14mm (vs. 18mm) (i.e. a 10% response). The patient developed a number of high-grade chemotherapy-related toxicities, including daily vomiting, fever, anemia, and thrombopenia requiring platelet transfusion. A second cycle of the same chemotherapy regimen was given, but with a 20% dose reduction. At that time, the patient had no abdominal pain and was able to interrupt analgesics. Radiological evaluation showed a partial response: 79mm (vs. 96mm), 60mm (vs. 84mm) and no adenopathy (i.e. a 40% response). The patient required another platelet transfusion but developed HLA alloimmunization, which limited the treatment options available. A third cycle of third-line chemotherapy was given at the same reduced dosages used in cycle 2. Radiological evaluation showed an 59% response: 52mm (vs. 79mm), 44mm (vs. 60mm) and no adenopathy (Figure 3). Unfortunately, the patient developed thrombopenia complications, including digestive tract and brain bleeding, resulting in death secondary to treatment toxicity.
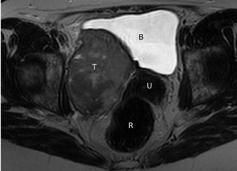
Figure 1
Figure 1
MRI performed during first-line chemotherapy.
T2-weighted transverse plane image showing the tumor (T) developed
extrinsically from the right lateral bladder wall. The tumor presents as a mass
with intermediate T2 signal, discreetly heterogeneous with spots with higher
signal considered as necrosis. B: bladder, U: uterus (cervix), R: rectum.
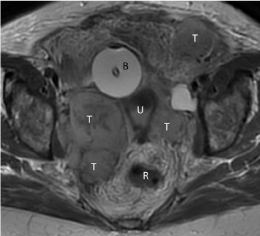
Figure 2
Figure 2
MRI showing recurrence after surgery.
T2-weighted transverse plane image showing the multiple tumor nodules (T),
including in the anterior left pelvic wall, right and left pelvic lateral walls, with
intermediate T2 signal. B: bladder (with a urinary catheter), U: uterus (cervix),
R: rectum.
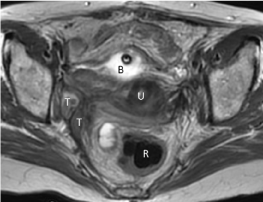
Figure 3
Figure 3
MRI showing a very good response after the addition of
bevacizumab to chemotherapy.
T2-weighted transverse plane image showing a single nodule remaining (T)
on the right pelvic lateral wall, having partly lost its intermediate T2 signal.
The anterior and left pelvic wall nodules have completely disappeared. B:
bladder (with a urinary catheter), U: uterus (cervix), R: rectum.
Discussion
Neuroendocrine bladder cancer is a very rare tumor and has poor prognosis. There is currently no large prospective clinical trial data and no standard treatment. Radiotherapy may be an option for palliative or curative care. Surgery is another approach, especially when the neuroendocrine tumor is associated with urothelial carcinoma of the bladder, although results are controversial. Chemotherapy is the main treatment, especially in the neoadjuvant setting, because neuroendocrine bladder cancer is very sensitive to chemotherapy and is often diagnosed at advanced stage: 90% of patients present with stage II disease and 25% are stage IV [1,5]. Chemotherapy regimens described in retrospective series for locally advanced (as neoadjuvant chemotherapy) or metastatic disease are: cisplatin and etoposide; ifosfamide, doxorubicin, etoposide and cisplatin; ifosfamide, etoposide and cisplatin; etoposide, cisplatin, cyclophosphamide, vincristine and doxorubicin; and methotrexate, vinblastine, cisplatin and doxorubicin [1,2,6,7]. There is one prospective phase II study in neuroendocrine bladder cancer [8]. It assessed the benefit of an alternating doublet of chemotherapy with ifosfamide plus doxorubicin and cisplatin plus etoposide during 4 cycles as neoadjuvant chemotherapy for resectable disease, or up to 2 cycles beyond maximal response for metastatic disease. Eighteen patients with locally advanced disease up to T4aN0M0 were enrolled, of whom 78% had a major response (defined as a down-staging up to pT1N0M0). Overall survival was 58 months. In the twelve enrolled patients who had metastatic disease, the overall response rate was nearly 100%; median overall survival was 13.3 months. Neutropenic fever occurred in 7/30 patients (23%) [8]. Bevacizumab has not been formally studied in neuroendocrine bladder cancer. However, over expression of the vascular endothelial growth factor receptor (VEGFR) on endothelial cells has been documented in this type of tumor [1]. For patients with advanced small cell lung cancer, adding bevacizumab to conventional first-line chemotherapy (cisplatin and etoposide) was associated with progression-free survival of 4.7 months, overall survival of 10.9 months and an overall response rate of 63.5% [9]. The most common adverse event was neutropenia, which occurred in 57.8% of patients [9]. Another randomized phase II study in advanced small cell lung cancer compared bevacizumab to placebo in combination to cisplatin or carboplatin plus etoposide as first-line therapy. Progression-free survival was 5.5 and 4.4 months, respectively, in the bevacizumab and placebo groups (hazard ratio 0.56, 95% confidence interval 0.32–0.86); corresponding values for median overall survival were 9.4 and 10.9 months, median duration of response were 4.7 and 3.2 months, and grade ≥3 toxicity were 75% and 60% [10]. Our patient showed a good response to bevacizmabcontaining chemotherapy, but unfortunately died from treatmentrelated toxicity. The number of platelet transfusions with pooled platelets appears to be the only risk factor for alloimmunization in this case. To date, there is no data to suggest that bevacizumab or the type of chemotherapy play a role in the development of anti-HLA antibodies. Abnormal signaling pathways that have been described in both small cell lung cancer and neuroendocrine cancer of the bladder include expression of c-kit, c-MYC amplification, EGFR mutations that activate RAS-RAF-MAPK pathways, over expression of BCL2, and TP53 mutation [1,6]. Over expression of p53 doesn’t appear to correlate with prognosis, while over expression of p16 may be an early and necessary event. Expression of p63 may be helpful for differentiating neuroendocrine bladder cancer from urothelial bladder cancer because it is inactivated in the former (92.8%) and expressed in the latter (81.3%) [11].
Conclusion
Although this patient died from treatment-related toxicity, she showed an almost complete response to third-line chemotherapy containing bevacizumab. Given the lack of data on treatments for neuroendocrine bladder cancer, our experience suggests that a phase II study assessing the benefit of adding bevacizumab to conventional chemotherapy in this setting appears to be warranted.
Acknowledgments
English language medical writing assistance was provided by Nicola Ryan, independent medical writer.
References
- Ismaili N. A rare bladder cancer--small cell carcinoma: review and update. 2011; 6: 75.
- Ismaili N, Heudel PE, Elkarak F, Kaikani W, Bajard A, Ismaili M, et al. Outcome of recurrent and metastatic small cell carcinoma of the bladder. 2009; 9: 4.
- Abrahams NA, Moran C, Reyes AO, Siefker-Radtke A, Ayala AG. Small cell carcinoma of the bladder: a contemporary clinicopathological study of 51 cases. 2005; 46: 57-63.
- Jordan JJ, Inga A, Conway K, Edmiston S, Carey LA, Wu L, et al. AlteredFunction p53 Missense Mutations Identified in Breast Cancers Can Have Subtle Effects on Transactivation. Mol Cancer Res. 2010 701-716.
- Lynch SP, Shen Y, Kamat A, Grossman HB, Shah JB, Millikan RE, et al. Neoadjuvant chemotherapy in small cell urothelial cancer improves pathologic down staging and long-term outcomes: results from a retrospective study at the MD Anderson Cancer Center. 2013; 64: 307-313.
- Pant-Purohit M, Lopez-Beltran A, Montironi R, MacLennan GT, Cheng L. Small cell carcinoma of the urinary bladder. 2010; 25: 217-221.
- Geynisman DM, Handorf E, Wong Y-N, Doyle J, Plimack ER, Horwitz EM, et al. Advanced small cell carcinoma of the bladder: clinical characteristics, treatment patterns and outcomes in 960 patients and comparison with urothelial carcinoma. 2015.
- Siefker-Radtke AO, Kamat AM, Grossman HB, Williams DL, Qiao W, Thall PF, et al. Phase II Clinical Trial of Neoadjuvant Alternating Doublet Chemotherapy With Ifosfamide/Doxorubicin and Etoposide/Cisplatin in Small-Cell Urothelial Cancer. J Clin Oncol. 2009; 2592–2597.
- Horn L, Dahlberg SE, Sandler AB, Dowlati A, Moore DF, Murren JR, et al. Phase II Study of Cisplatin Plus Etoposide and Bevacizumab for Previously Untreated, Extensive-Stage Small-Cell Lung Cancer: Eastern Cooperative Oncology Group Study E3501. J Clin Oncol. 2009; 6006–6011.
- Spigel DR, Townley PM, Waterhouse DM, Fang L, Adiguzel I, Huang JE, et al. Randomized Phase II Study of Bevacizumab in Combination With Chemotherapy in Previously Untreated Extensive-Stage Small-Cell Lung Cancer: Results From the SALUTE Trial. J Clin Oncol. 2011; 2215–2222.
- Zhao X, Flynn EA. Small cell carcinoma of the urinary bladder: a rare, aggressive neuroendocrine malignancy. Arch Pathol Lab Med. 2012; 136: 1451-1459.