Review Article
Cyclin D1 Expression in Triple-Negative Breast Cancer with New Treatment Implications
Hartel PH1*, Donald R2 and Fleming3
1Davis Medical Center of Davis Health System, Elkins, West Virginia, USA
2West Virginia School of Medicine, Morgantown, West Virginia, USA
3West Virginia School of Osteopathic Medicine, Lewisburg, West Virginia, USA
*Corresponding author: Paul H Hartel, Medical Director, Pathology and Laboratory Medicine, Davis Medical Center, Reed St and Gorman Ave, Elkins WV 26241,USA
Published: 29 Jul, 2016
Cite this article as: Hartel PH, Donald R, Fleming. Cyclin
D1 Expression in Triple-Negative
Breast Cancer with New Treatment
Implications. Clin Oncol. 2016; 1: 1044.
Abstract
While targeted therapies are available for breast cancer patients whose tumors express ER,
PR, or show HER-2 overexpression, no such treatment exists for “triple-negative” cases. As
immunohistiochemistry (IHC) expression of Cyclin D1 has been shown concordant with Cyclin D1
gene amplification, we evaluated Cyclin D1 IHC expression in 25 cases of triple-negative invasive
ductal carcinoma to clarify its relationship with clinical and pathologic parameters. Twenty-six
invasive carcinomas negative for ER, PR, and HER-2 were reviewed. One sarcomatoid carcinoma
was omitted leaving 25 invasive ductal carcinomas for this pilot study. Clinical information,
including treatment response, was gleaned from patient records. Only one case was known to be
BRCA-positive. Tumors were morphologically reviewed and Cyclin D1 IHC applied using BCL-1.
Chi square and t-test statistical analyses were performed. Patients were female and ranged in age
from 32 to 88 years (m=59). Tumors were in left (n=16) and right breasts (n=9) with tumor size
ranging from 0.4 cm up to 7.2 cm (m=2.7cm). Tumors were invasive dustal carcinomas of no special
type, Nottingham grade 1 (n=1), 2 (n=10) and 3 (n=14). Eight cases had an in-situ component, high
grade (n=7) or low grade (n=1), ranging from 5% to 70% of tumor (m= 23%). All tumors showed
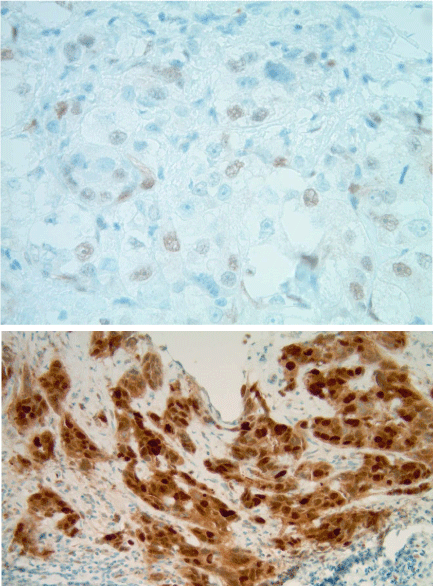
Cyclin D1 expression from light and focal staining (1+; n=6; Group 1) to focal intense (2+; n=9) or
diffuse intense staining (3+; n=10). The latter two groups were combined for analyses (Group 2).
While patients were younger in Group 2, this was not statistically significant. Patients with tumors
showing intense BCL-1 staining (Group 2) had larger tumors (p<.05) with more capillary/lymphatic
invasion (p<.005) and lymph node metastases (p<.007), and were less likely to respond to treatment
(p=.01; see Table 1). Cyclin D1 expression may serve as a marker for more biologically aggressive
triple-negative breast cancer. These tumors may respond to targeted therapy that down-regulates
Cyclin D1 amplification. Further research with larger sample sizes is needed.
Keywords: Triple negative; Breast cancer; Invasive ductal carcinoma; Cyclin D1;
Immunohistochemistry; Personalized medicine
Introduction
Triple-negative breast cancer, defined as ER-negative, PR-negative, and lacking HER-2neu overexpression, accounts for approximately 15% of breast cancers and typically affects patients younger than 50 years of age, is more prevalent in African–American women, and is more aggressive than other breast cancers [1,2]. Currently, these patients do not benefit from effective targeted therapies in the age of personalized medicine. Only a subset of patients benefits from systemic chemotherapy, while most patients show increased risk of recurrence early in the disease course, and increased mortality in the first 5 years following treatment [3]. Furthermore, the identification of reliable biomarkers to help select high and low risk subsets of patients and guide treatment has been elusive, and markers to date, primarily immunohistochemical, have been prognostically inconsistent. We evaluated a small sample of ER-, PR-, and HER2neu-negative invasive ductal carcinomas of no special type to investigate the relationship between Cyclin D1 tumor expression and clinical and pathologic parameters. Twenty-five tumors were morphologically reviewed and Cyclin D1 immunohistochemistry applied using BCL-1. Patients with tumors showing intense BCL- 1 staining had larger tumors with more capillary/lymphatic invasion and lymph node metastases, and were less likely to respond to treatment. We believe Cyclin D1 expression may serve as a robust biomarker for more aggressive triple-negative breast cancer, and that targeted therapy that downregulates Cyclin D1 amplification may hold potential treatment promise for these patients [4,5].
Figure 1
Methods
Twenty-six invasive carcinomas, negative for ER, PR, and HER-2 were reviewed from our files. One sarcomatoid carcinoma was omitted leaving 25 invasive ductal carcinomas of no special type for study. Clinical information, including treatment response, was gleaned from patient electronic records and hematologist/ oncologist assessment (DF). Only one case was BRCA-positive. Specimens included mastectomies, lumpectomies, and needle biopsies [3]. Tumors were morphologically reviewed by a surgical pathologist with interest in breast pathology (PHH) and Cyclin D1 immunohistochemistry applied using BCL-1. Tumors showing BCL-1 staining in greater than or equal to 80% of tumor cells were considered to show diffuse staining, while tumors showing BCL-1 staining in less than or equal to 20% of tumor cells were considered to show focal staining. Intensity of staining was either 1+ (light) or 3+ (intense). Chi square and t-test statistical analyses were performed on categorical and continuous data, respectively.
Results
Patients were female and ranged in age from 32 to 88 years (m=59). Tumors were in left (n=16) and right breasts (n=9) with tumor size ranging from 0.4cm up to 7.2 cm (m=2.7cm). Tumors were invasive ductal carcinomas of no special type, Nottingham grade 1 (n=1), 2 (n=10) and 3 (n=14) predominantly with solid nests and cords of tumor cells, large irregular and vesicular nuclei with prominent nucleoli, numerous mitoses, and focal necrosis. Eight cases had an in-situ component, high grade (n=7) or low grade (n=1), ranging from 5% to 70% of tumor (m= 23%). All tumors showed Cyclin D1 expression from light and focal staining (1+; n=6; Group 1) to focal intense (2+; n=9) or diffuse intense staining (3+; n=10; Figure 1). The latter two groups were combined for analyses (Group 2). While patients were younger in Group 2, this was not statistically significant. Patients with tumors showing intense BCL-1 staining (Group 2) had larger tumors (p<.05) with more capillary/lymphatic invasion (p<.005) and lymph node metastases (p<.007), and were less likely to respond to treatment (p=.01; see Table 1).
Table 1
Discussion
Patients with “triple negative” breast cancer do not currently benefit from targeted therapies in the age of personalized medicine. We evaluated a small sample of ER-, PR-, and HER2neu-negative invasive ductal carcinomas to investigate the relationship between Cyclin D1 tumor expression and clinical and pathologic variables. Cyclin D1 amplification is known to exist in many cancers, including breast cancer, and plays a role in carcinogenesis [6]. Cyclin D1 locus amplification has been shown to exhibit near complete concordance with immunohistiochemical protein expression [7]. While larger sample sizes are needed, we believe that, based on our results, Cyclin D1 may serve as a potent biomarker identifying a subgroup of triple-negative cancers with poor prognostic indicators, and may serve as a treatment target for affected patients [4,5]. Much of the recent research on triple-negative breast cancer has focused on subclassifying basal-like and non-basal-like tumors. Basal-like tumors comprise a heterogeneous group, similar to triple-negative breast cancer, that accounts for up to 15% of all breast cancers, affect younger patients, are more prevalent in African-American women, and are more aggressive [1]. Histologically, the majority of basallike breast cancers are invasive ductal carcinomas of no special type, have high nuclear grade and high mitotic counts, focal necrosis, pushing borders, conspicuous lymphocytic infiltrate, and sometimes medullary features [8-10]. While there is no accepted international definition of “basal-like” breast cancer, the majority of basal-like breast cancers lack ER, PR, and HER2 protein over expression, whereas they express genes and proteins usually found in basal (myoepithelial) cells of the normal breast, most notably including high-molecular-weight cytokeratins (CK5/6, 14 and 17) [9,11-14]. Immunohistochemical expression of p53 or TP53 gene mutations are observed in up to 85% of cases [15,16], and alterations of the pRB and p16 G1/S constituents of the cell-cycle are prevalent [17]. Basal-like cancers also share high proliferation indices as defined by mitotic counting or by Ki67 immunohistochemistry [17]. Overall, however, prognostic results have been variable, with some studies finding basal-like tumors to have poorer prognosis [1,3,18], while others suggest a better prognosis with some variables compared with non-basal like triple-negative cancers [19]. These differences tend to be a function of how basal-like tumors are defined, for example, by histology, immunohistochemistry, or gene expression. A recent study compared triple-negative breast cancers defined as basal-like by all three parameters, ie, histology, immunohistochemistry, and genomics [19]. Basal-like triple-negative breast cancers defined by either EGFR and/or CK5/6 immunoreactivity showed an increase risk of death within 15 years compared with tumors classified as basal-like due to morphologic features such as solid growth pattern, pushing margin, and necrosis. In contrast, those tumors classified as basal-like based on gene expression [20] showed a significantly better 15-year survival than other groups. Such discordant findings highlight the heterogeneity within triple-negative breast cancers when evaluated according to the presence or absence of basal-like features, and raise questions as to the clinical utility of this designation. We found that all of our triple-negative breast cancers, similar in morphology, all expressed Cyclin D1 although with variable intensity. Our results showed a consistent relationship with Cyclin D1 immunoreactivity and tumor size, the presence of prognostic indicators such as capillary/lymphatic invasion and lymph node metastases, and treatment response. We believe that Cyclin D1 may serve as a more robust and consistent biomarker for aggressive triple-negative breast cancers than classification based on presence or absence of basal-like features, and may serve as a useful treatment target in this patient population. Future work must duplicate the present findings with larger study samples.
References
- Thike AA, Iqbal J, Cheok PY, Chong AP, Tse GM, Tan B, et al. Triple negative breast cancer: outcome correlation with immunohistochemical detection of basal markers. Am J Surg Pathol. 2010; 34: 956-964.
- Bauer KR, Brown M, Cress RD. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California Cancer Registry. Cancer. 2007; 109: 1721–1728.
- Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-Negative Breast Cancer: Clinical Features and Patterns of Recurrence. Clin Cancer Res. 2007; 13: 4429-4434.
- Finn RS, Bengala C, Ibrahim N, Roché H, Sparano J, Strauss LC, et al. Dasatinib as a single agent in triple-negative breast cancer: results of an open-label phase 2 study. Clin Cancer Res. 2011; 17: 6905-6913.
- Finn RS, Dering J, Ginther C, Wilson CA, Glaspy P, Tchekmedyian N, et al. Dasatinib, an orally active small molecule inhibitor of both the src and abl kinases, selectively inhibits growth of basal-type/"triple-negative" breast cancer cell lines growing in vitro. Breast Cancer Res Treat. 2007; 105: 319-326. Epub 2007 Feb 1.
- Withers DA, Harvey RC, Faust JB, Melnyk O, Carey K, Meeker. TC Characterization of a candidate bcl-1 gene. Mol Cell Biol. 1991; 11: 4846-4853.
- Fracchiolla NS, Pruneri G, Pignataro L, Carboni N, Capaccio P, Boletini A, et al. Molecular and immunohistochemical analysis of the bcl-1/cyclin D1 gene in laryngeal squamous cell carcinomas: correlation of protein expression with lymph node metastases and advanced clinical stage. Cancer. 1997 Mar 15; 79: 1114-1121.
- Livasy CA, Karaca G, Nanda R. Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Mod Pathol. 2006; 19: 264–271.
- Fulford LG, Easton DF, Reis-Filho JS. Specific morphological features predictive for the basal phenotype in grade 3 invasive ductal carcinoma of breast. Histopathology. 2006; 49: 22–34.
- Turner NC, Reis-Filho JS, Russell AM. BRCA1 dysfunction in sporadic basal-like breast cancer. Oncogene. 2007; 26: 2126–2132.
- Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004; 10: 5367-5374.
- Cheang MC, Voduc D, Bajdik C. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008; 14: 1368–1376.
- Fulford LG, Reis-Filho JS, Ryder K. Basal-like grade III invasive ductal carcinoma of the breast: patterns of metastasis and long-term survival. Breast Cancer Res. 2007; 9: R4.
- Reis-Filho J, Pinheiro C, Lambros M. EGFR amplification and lack of activating mutations in metaplastic breast carcinomas. J Pathol. 2006; 209: 445–453.
- Rakha E, Ellis I, Reis-Filho J. Are triple-negative and basal-like breast cancer synonymous? Clin Cancer Res. 2008; 14: 618–619.
- Reis-Filho JS, Savage K, Lambros MB. Cyclin D1 protein overexpression and CCND1 amplification in breast carcinomas: an immunohistochemical and chromogenic in situ hybridisation analysis. Mod Pathol. 2006; 19: 999–1009.
- Subhawong AP, Subhawong T, Nassar H. Most basal-like breast carcinomas demonstrate the same Rb−/p16+ immunophenotype as the HPV-related poorly differentiated squamous cell carcinomas which they resemble morphologically. Am J Surg Pathol. 2009; 33: 163–175.
- Thike AA, Cheok PY, Jara-Lazaro AR, Tan B, Tan P, Tan PH. Triple-negative breast cancer: clinicopathological characteristics and relationship with basal-like breast cancer. Mod Pathol. 2010; 23: 123-133.
- Gazinska P, Grigoriadis A, Brown JP, Millis RR, Mera A, Gillett CE, et al. Comparison of basal-like triple-negative breast cancer defined by morphology, immunohistochemistry and transcriptional profiles. Mod Pathol. 2013; 26: 955-966.
- Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009; 27: 1160-1167.