Review Article
A Modified Decompression and Bone Graft Technique for the Treatment of Avascular Necrosis of the Femoral Head
Shehadeh AM1, El Al SA1, Salem A2*, Jafar A1, Shahin IA1, Omar M1 and Albtoush3
1Department of Surgery- Section of Orthopaedic Surgery, King Hussein Cancer Center, Jordan
2Department of Radiation Oncology, University of Manchester, UK
3Department of Diagnostic Radiology, King Hussein Cancer Center, Jordan
*Corresponding author: Ahmad Salem, University of Manchester, Wolfson Molecular Imaging Centre, Manchester, M20 3LJ, UK
Published: 15 Jul, 2016
Cite this article as: Shehadeh AM, El Al SA, Salem A,
Jafar A, Shahin IA, Omar M, et al. A
Modified Decompression and Bone
Graft Technique for the Treatment of
Avascular Necrosis of the Femoral
Head. Clin Oncol. 2016; 1: 1036.
Abstract
Objectives: Avascular necrosis (AVN) of the femoral head is a pathologic process resulting from
interruption of blood supply to bone. The aim of this article is to describe the technical aspects and
outcome of a modified technique of core decompression and bone graft injection for the treatment
of AVNFH.
Methods: Twenty patients (26 femoral head AVN) Ficat stage I to early III were treated using core
decompression kit followed by injection with bone graft material. Nine hips were stage III, 16 stages
II and 1 stage I. Average operative time was 25 minutes.
Results: At a median follow-up of 48 months, 20 hips (77%) had almost complete pain relief
while pain persisted in 6 hips (23%). All patients who demonstrated clinical response exhibited
radiological stabilization of disease. The mean Haris hip score for all patients’ prior and following
surgery was 41 and 85, respectively (p<0.0001).
Conclusions: Femur head decompression using core decompression kit followed by bone substitute
injection can result in long-term pain relief and prevention of progression of AVN in the majority
of patients.
Keywords: Femoral; Head; Decompression; Bone graft; Avascular necrosis
Introduction
Avascular necrosis of the femoral head (AVNFH) is a debilitating disease affecting patients
in the fourth and fifth decade [1-4]. It can be idiopathic or secondary; the most common causes
include steroid and alcohol intake. Steroid intake is most commonly encountered in patients
following renal transplants, lupus erythematosus, asthma, glomerulonephritis, peripheral neuritis,
sinusitis, pemphigus, Guillain-Barre syndrome, head injuries and those receiving combination
chemotherapy [5]. A number of treatment options are available for this disease. Non-surgical
management includes medical treatment, protected weight bearing and electrical stimulation.
Surgical management includes core decompression, debridement and grafting and arthroplasty [4].
An understanding of the natural history of AVNFH is important for predicting the fate of the hip,
in choosing the appropriate treatment and in evaluating the results of various treatments. The rate
at which the femoral head will collapse is related among other things to the cause of disease, the
stage and extent of disease at the initial diagnosis and to the size and location of the necrotic lesion.
Nevertheless, very small lesions (<15% involvement of the femoral head) may remain minimally
symptomatic without any formal treatment. On the other hand, large lesions (>50% involvement
of the femoral head) continually progress to collapse and arthrosis in greater than 85% of cases
[6]. Numerous reviews of the natural progression in patients who were treated non-surgically with
crutches and partial non-weight bearing ambulation document a risk of progression of 85-92% [4].
Core decompression, which is minimally invasive and lacks complexity when compared to another
surgical option, is a commonly performed procedure for AVNFH. Typically, this is recommended
for early-stage pre-collapsed osteonecrosis with small to medium size lesions.
In this prospective study, we describe the technical aspects and outcome of a modified technique
of core decompression and bone graft injection for the treatment of AVNFH.
Methods
We prospectively evaluated the outcome of 26 hips in 20 patients with AVNFH who received
treatment at King Hussein Cancer Centre (Amman, Jordan) from
January 2009 to August 2013 using a modified technique for core
decompression and backfilling with injectable bone graft. All
patients signed an informed consent and this study was approved
by the Institutional Review Board. Patients were staged by one
musculoskeletal radiologist (OMA) utilizing anterior posterior (AP)
and frog lateral radiographs and magnetic resonance imaging (MRI).
Staging was based on Ficat and Arlet staging system (stage I-IV) [7].
Participants were cancer patients who received corticosteroids as part
of their chemotherapy treatment protocol. The median cumulative
dose of Dexamethasone was 1,438mg (range; 274mg to 8,389mg).
Decompression was performed for patients with symptomatic
stage I, all patients with stage II and patients with stage III with early
collapse (crescent sign or early flattening). There were 9 females (14
hips) and 11 males (12 hips); the mean age at time of procedure was
22.1 years (range; 8 to 54 years).
Clinical follow-up was according to the Haris Hip Scroe (HHS)
determined preoperatively and postoperatively. Serial AP and
frog lateral radiograph views of the hips were obtained 6 weeks
postoperatively and then every 3 months. Failure of treatment was
defined as lack of 10 grades increase in HHS after procedure and/or
persistence of pain. Paired t-test was used to compare postoperative
HHS to preoperative HHS; a p-value of ≤0.05 was considered to
indicate a statistically significant difference.
Surgical technique
We utilized a specialized core decompression kit (Wright Medical
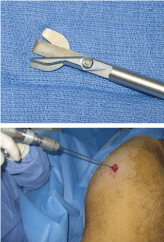
Technology Inc., Arlington,TN); (Figure 1). In addition, we made use
of the X-Ream Percutaneous Expandable Reamer (Wright Medical
Technology Inc., Arlington,TN), which is a specialized reamer with
an expanding tip; (Figure 2A and B).
The following are the steps involved in this procedure;
1. Patient placed at lateral position with operative side up
2. Cleaning and draping of the operative limb with full exposure
to the hip and thigh
3. A 1.5cm stab incision is made over the lateral aspect of
proximal femur 2cm below the ridge of greater trochanter
4. Dissection till reaching the bone surface
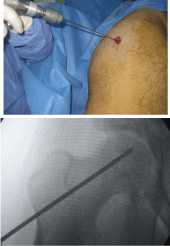
5. A 3.2mm fluted guide wire is advanced under fluoroscopic
guidance from an entry site just distal to the greater trochanter up
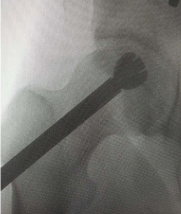
the femoral neck into the necrotic lesion in the femoral head; (Figure
3A and B)
6. A tissue protector is inserted till it touches the bone and then
the 9mm cannulated drill is inserted to start decompression of the
necrotic region
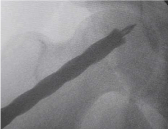
7. Drilling should be 5mm away from the articular surface;
(Figure 4)
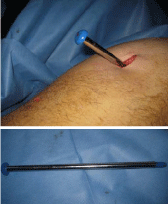
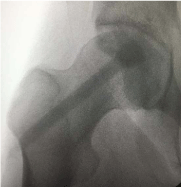
8. Removal of the drill and the guide wire and insertion of the
working cannula into the track; (Figure 5A and B)
9. A special curette is used to remove all debris which is then sent
for histopathlological examination
7. The X-Ream Percutaneous Expandable Reamer is inserted to
remove a greater volume
of the necrotic bone, the blade control knob is turned clockwise
until it cannot be turned anymore and the entire reamer is rotated
once or twice or three times till the resistance is high the instrument
cannot be further rotated
8. The control knob is then turned clockwise again and step
8 is repeated till reaching the full expansion of the reamer and the
marker at the control knob is reading number 3; corresponding to the
maximum expansion which is 2.1 cm; (Figure 6)
9. AP and lateral radiological control is mandatory all through
the reaming procedure to verify the location and extent of the reamer
expansion and make sure that no breach of the joint has occurred
10. If there was a concern regarding the penetration into the joint,
Angiograffin contrast is to be injected into the track to see if there is
spillage of the contrast into the hip joint
11. Turning the blade control knob counter clockwise till full
collapse and removal of the X-Ream and curettage again to remove
all debris and final irrigation and suction of the track
12. The generated track is ready now for injection of bone graft
13. The core track is now backfilled with graft material; MIIG 115
(Wright Medical
Technology Inc., Arlington, TN); (Figure 7)
Following the operation, patients were allowed to start walking
full weight bearing as tolerated the first day after surgery.
Figure 1
Figure 2A and B
Figure 3A and B
Figure 4
Figure 5A and B
Figure 6
Figure 7
Results
All patients who received treatment were symptomatic with hip
pain; stage I (1 hip), stage II (13 hips) and stage III (12 hips). All
patients were reviewed at a median follow up period of 43 months,
(range; 10-60 months). No complications related to the procedure
were reported.
The median preoperative HHS was 41 (range; 30 to 82), the
median postoperative HHS at last follow up was 80 (range; 40 to 96);
indicating a significant difference between scores (p-value <0.0001).
There were no significant differences between stages I, II and III in
terms of HHS increase following the operation; table 1. In terms of
symptomatic response, pain resolved in 20 hips (77%) and persisted
in 6 hips (23%).
Radiological evaluation revealed that 14 hips (54%) were
radiologically stable at last follow up; table 2. Clinical failure was
encountered in 6 hips (23%); 4 of which were converted to hip
arthroplasty. Overall, 15% of all hips were converted to arthroplasty;
three patients were stage III disease at time of operation and
progressed to stage IV, one patient was stage II at time of operation
and progressed to stage IV after procedure.
Table 1
Table 2
Table 3
Discussion
The use of this core decompression technique offers a number of
advantages over traditional core decompression methods including
simplicity of the technique, the minimally invasive approach and the
more extensive percutaneous debridement of necrotic lesions [8,9].
By utilizing the X-REAM, we can enhance the area of debridement at
the femoral head through the same core width that is created at the
femur neck and lateral femoral cortex. Core decompression alone can
increase the structural compromise of the subchondal plate and can
increase the risk of femur head collapse. This technique penetrates the
body of necrotic lesions and as such, places the head at greater risk of
structural collapse compared to the untreated situation [10,11].
Our selection of MIIG 115 (calcium sulphate) was based on our
knowledge that this product can provide a maximum compressive
strength of 15 MPa, as compared to 4 MPa, the compressive strength
of cancellous bone, within 2 hours after injection. Essentially, this
enables immediate post-operative weight bearing by the patient.
Furthermore, this product is typically completely reabsorbed in
6-12 weeks; if accidental spillage into the hip joint occurs, it will be
resorbed within 4 weeks with no need for arthortomy to take it out.
This is in variance to products that contains calcium phosphates [12-
14].
A literature review demonstrates that this technique has a
comparable outcome to more complicated procedures such as
vascularized bone graft insertion at the femur head and harvesting
and preparation of bone marrow aspirate; table 3. These procedures
are more time consuming, require longer hospital stay and are
associated potential donor site morbidity. The ideal graft material;
however, is still a matter of debate that should be further investigated
by comparative prospective studies.
References
- Etienne G, Mont M, Ragland PS. The diagnosis and treatment of non-traumatic osteonecrosis of the femoral head. Instru Course Lect. 2004; 53: 67-85.
- Liberman JR, Berfry DJ, Mont MA, Aaron RK, Callaghan JJ, Rajadhyaksha AD, et al. Osteonecrosis of the hip: management in the 21st century. Instr Course Lect. 2003; 52: 337-355.
- Mont MA, Jones IC, Einhorn TA, Hungerford DS, Reddi AH. Osteonecrosis of the femoral head .Potential treatment with growth and differentiation factors. Clin Orhop Relat Res. 1998; 355: S 314-335.
- Shannon BD, Trousdale RT. Femoral Osteotomies for Avascular Necrosis of the Femoral Head. Clin Orthop. 2004; 418: 34-40.
- Nixon JE. Early diagnosis and treatment of steroid induced avascular necrosis of bone. British Medical Journal. 1984; 288.
- Yoshioka T, Mishima H, Akaogi H, Sakai S, Li M, Ochiai N. Concentrated autologous bone marrow aspirate transplantation treatment for steroids induced osteonecrosis of femoral head in systemic lupus erythematosus. Int Orthop. 2011; 35: 823-829.
- Mont MA, Marulanda GA, Jones LC, Saleh KJ, Gordon N, Hungerford DS, et al. Systematic analysis of classification systems for osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006; 88: 16–26.
- Steinberg ME, Larcom PG, Strafford B, Hosick WB, Corces A, Bands RE, et al. Core decompression with bone grafting for osteonecrosis of the femoral head. Clin Orthop Relat Res. 2001; 386: 71–78.
- Petrigliano FA, Lieberman JR. Osteonecrosis of the hip: novel approaches to evaluation and treatment. Clin Orthop Relat Res. 2007; 465: 53–62.
- Penix AR, Cook SD, Skinner HB, Weinstein AM, Haddad RJ Jr. Femoral head stresses following cortical bone grafting for aseptic necrosis. A finite element study. Clin Orthop. 1983; 173: 159–165.
- Brown TD, Pedersen DR, Baker KJ, Brand RA. Mechanical consequences of core drilling and bone-grafting on osteonecrosis of the femoral head. J Bone Joint Surg Am.1993; 75: 1358–1367.
- Lee. Average cancellous bone values range from 0.5 to 11 MPa. Clin Orthop Rel Res 1991; 273: 177-183.
- Kelly CM, Wilkins RM, Gitelis S, Hartjen C, Watson JT, Kim PT. The Use of a Surgical Grade Calcium Sulfate as a Bone Graft Substitute. Clin Orthop Rel Res. 2001; 382: 42-50.
- Mirzayan R, Panossian V, Avedian R, Forrester DM, Menendez LR. The Use of Calcium Sulfate in the Treatment of Benign Bone Lesions. J Bone Joint Surg. 2001; 83A: 355-358.
- Liberman JR, Conduah A, Urist MR. Treatment of osteonecrosis the femoral head with core decompression and human BMP. Clin Orthop Relat Res. 2004; 429: 139-145.
- Mont MA, Etienne G, Ragland PS. Outcome of non vascularized bone grafting for osteonecrosis of the femoral head .Clin Orthop Relat Res. 2003; 417: 84-92.
- Cuervas-Mons M, Narbona J, Laguna R, Vaquero J. Autologous concentrated bone marrow graft in the tratment of femoral head AVN: clinical outcome after two years of follow up in a non-controlled prospective study. Rev Esp Cir Ortop Traumatol. 2013; 57: 106-110.
- Martin JR, Houdek MT, Sierra RJ. Use of concentrated bone marrow aspirate and platelet rich plasma during M.I decompression of the femoral head in the treatment of osteonecrosis. Croat Med J. 2013; 54: 219-224.
- Yoo MC, Kim K, Hahn CS, Parvizi J. Long term follow up of vascularized fibular grafting for femoral head necrosis. Clin Orthop Rela Res. 2008; 466: 133-140.