Editorial
Role of Gut Microbiota in Colorectal Cancer
Makker PN, Goyal S, Yu Y, Farhana L and Majumdar AN*
Department of Internal Medicine, Wayne State University, USA
*Corresponding author: Adhip N. Majumdar, Department of Internal Medicine, VA Medical Center, Research Service, Wayne State University, 4646 John R, Room-B4238, Detroit, MI 48201, USA
Published: 13 Jul, 2016
Cite this article as: Makker PN, Goyal S, Yu Y, Farhana L,
Majumdar AN. Role of Gut Microbiota
in Colorectal Cancer. Clin Oncol. 2016;
1: 1027.
Keywords
Colorectal cancer; Microbiome; Bile acids
Editorial
Colorectal cancer (CRC), an age-related malignancy whose incidence increases markedly after
the age of 50 years, is the third most common cancer worldwide and shows significant variations
in the distribution globally [1,2]. More than 1.2 million new cases of colorectal cancer (CRC) are
reported each year, most of which (~85%) occur sporadically as a result of the accumulation of
mutations and epigenetic modifications in several genes [3]. Incidence of CRC is found to vary
markedly worldwide, with 4.1 cases per 100,000 males in India to 59.1 cases in Czech Republic.
While among females, it ranges from 3.6 in India to 39.5 in New Zealand [1]. Some of the risk
factors for colorectal cancers include obesity, a diet low in fruits and vegetables, physical inactivity
and smoking [4]. There has been a decrease in the CRC mortality worldwide whereas the incidences
are on the rise [1]. The decline in CRC deaths is attributed to an advanced diagnostic and prognostic
technology, while, the “Westernized” life style in developing countries as well as improved longevity
in developed countries, contributes to a greater incidence of CRC [1]. A better understanding of the
environmental and other factor(s) that may be responsible for the increased incidence of CRC is
crucial for developing preventive strategies.
CRC is a multi-step process resulting from accumulation of mutations during progression from
normal epithelium to carcinoma. Genetic changes that occur at different stages of epithelial cell
carcinoma have been extensively studied by Fearon and Vogelstein in human colon cancer [5], and
have been reviewed by others. Briefly, two models have been proposed to explain the occurrence
of CRC. One model states that the initial step begins with somatic mutations in adenomatous
polyposis coli (APC) gene, which is considered as the initiating step of transforming the normal
mucosa to an adenoma (class I) by hyper-proliferation [6]. The hyper-proliferation is brought about
by accumulation of β-catenin that in turn enters the nucleus to trigger cell cycle [7]. The next step
involved is the activation of K-ras, which is a proto-oncogene that results in the transformation
of an early adenoma to an intermediate adenoma (class II adenoma) [6]. The third step is the
loss of function gene- deleted in colorectal cancer (DCC) gene on chromosome 18q resulting in
the formation of a class III adenoma [7]. The last step is the mutations in p53 gene that finally
transforms an adenoma into an invasive/early cancer [6]. It is predicted that the above 4 steps
take approximately 10 years and hence a 10 years interval was selected as the screening interval
for colonoscopies in people with normal colonic mucosa at initial colonoscopy [7]. The second
CRC model is based on “Microsatellite Instability” that causes mutations in DNA mismatch repair
genes leading to accumulation of uncorrected replication errors resulting in hyper proliferation and
eventually carcinoma [7].
It is becoming increasingly evident that the human intestinal microbiota may contribute to
the etiology of CRC [3]. The human colon harbors a complex microbial flora. Bacterial density in
human colon is among the highest found in nature, approaching 1012 bacteria/gm wet weight of
feces. Given the sheer vastness of our microflora and numerous arrays of species, interactions, and
metabolites produced, bacteria are likely pivotal players in several gastrointestinal diseases including
CRC [8-10]. Predominant bacterial phyla associated with adenomas and CRC are Bacteriodetes and
Firmicutes (family Lachnospiraceae including Clostridium, Ruminococcus and Butyrivibrio) [11].
These two phyla also contribute to 95% of the total GI ecosystem [12]. One of the primary roles
of gut bacteria is to participate in biotransformation of products in the gut, which among others
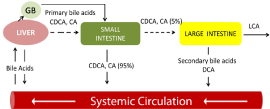
include bile acids secreted from the liver, as depicted in Figure 1.
Primary bile acids are synthesized from cholesterol in hepatocytes via cholesterol 7-α-hydroxylase.
In the large intestine, they are deconjugated by 7-α-dehydroxylation by enteric bacteria to form the
secondary bile acids. Among the secondary bile acids deoxycholic acid (DCA) and lithocholic acid
(LCA), are thought to be most notorious for their co-carcinogenic
activity [13-15] and considered to be most significant with respect
to the development of CRC [16-18]. In support of this postulation,
others have reported that cholecystectomy, which increases bile acids
in the colon, leads to advanced colon adenomas and CRC [19-22].
Our published and unpublished observations also suggest a role for
gut microbiome and their metabolites, the bile acids, specifically DCA
and LCA in promoting colon carcinogenesis. We have observed that
African Americans, who are known to have a higher incidence of CRC
than Caucasian Americans [23,24], also show a significant increase
in the number of adenomas [25]. These increases are associated with
a concomitant rise in pro-inflammatory Fusobacterium nucleatum,
Enterobacterium and clostridium, which are associated with CRC
[26,27] and the levels of DCA and LCA as well as self-renewing,
chemo-resistant, pluripotent cancer stem cells ([25] and unpublished
observations). Latter are known to play a pivotal role in the
development and progression of many malignancies, including CRC
[28,29]. We also observed that both DCA and LCA enhanced cancer
stem cells in colonic mucosal cells (unpublished observations).
Further support for DCA and/LCA-induction of colon
carcinogenesis comes from in vitro cell culture and in vivo animal
experiments. Data from several in vitro and in vivo studies revealed
that exposure of normal colonic epithelial cells to DCA caused
mitotic aberrations that are known to be precursors of aneuploidy
and are indicators of genome instability [16,17]. Studies with rats
have demonstrated that administration of bile acids, specifically DCA
greatly enhanced the incidence of tumors when a potent carcinogen
was also administered [13], indicating tumor promoter activity of bile
acids.
Clearly, these and other studies suggest that intestinal microbiota
contributes to the etiology of colorectal cancer via the pro-carcinogenic
activities of specific pathogens. Further studies are undoubtedly
needed to determine the precise role of different microbiota in the
colon in the development of progression of colorectal cancer.
Figure 1
Figure 1
Systemic/Enterohepatic Circulation of Bile Acids: Approximately
5% of primary bile acids pass on to the large intestine, where they may be
transformed into the potential toxins DCA and LCA. DCA= Deoxycholic Acid;
LCA=Lithocholic Acid; CDCA=Chenodeoxycholic Acid. CA=Cholic Acid
[Adapted from Plotnikoff GA; Glob Adv Health Med 3:33-43].
Acknowledgment
The work was supported by grants to Dr. Majumdar from the NIH/NCI (1R21CA175916), the Department of Veteran Affairs (I101BX001927) and the Metropolitan Detroit Research and Education Fund (MDREF).
References
- Center MM, Jemal A, Ward E. International trends in colorectal cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2009; 18: 1688-1694.
- Parkin DM. International variation. Oncogene. 2004; 23: 6329-6340.
- Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014; 12: 661-672.
- Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P. Smoking and colorectal cancer: a meta-analysis. JAMA. 2008; 300: 2765-2778.
- Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990; 61: 759-767.
- Todaro M, Francipane MG, Medema JP, Stassi G. Colon cancer stem cells: promise of targeted therapy. Gastroenterology. 2010; 138: 2151-2162.
- Markle B, May EJ, Majumdar AP. Do nutraceutics play a role in the prevention and treatment of colorectal cancer? Cancer Metastasis Rev. 2010; 29: 395-404.
- McGarr SE, Ridlon JM, Hylemon PB. Diet, anaerobic bacterial metabolism, and colon cancer: a review of the literature. J Clin Gastroenterol. 2005; 39: 98-109.
- Keku TO, Dulal S, Deveaux A, Jovov B, Han X. The gastrointestinal microbiota and colorectal cancer. Am J Physiol Gastrointest Liver Physiol. 2015; 308: G351-363.
- Winter TA, O'Keefe SJ, Callanan M, Marks T. Effect of severe undernutrition and subsequent refeeding on gut mucosal protein fractional synthesis in human subjects. Nutrition. 2007; 23: 29-35.
- Dejea CM, Wick EC, Hechenbleikner EM, White JR, Mark Welch JL, Rossetti BJ, et al. Microbiota organization is a distinct feature of proximal colorectal cancers. Proc Natl Acad Sci U S A. 2014; 111: 18321-18326.
- Allen-Vercoe E, Jobin C. Fusobacterium and Enterobacteriaceae: important players for CRC? Immunol Lett. 2014; 162: 54-61.
- Narisawa T, Magadia NE, Weisburger JH, Wynder EL. Promoting effect of bile acids on colon carcinogenesis after intrarectal instillation of N-methyl-N'-nitro-N-nitrosoguanidine in rats. J Natl Cancer Inst. 1974; 53: 1093-1097.
- Reddy BS, Wynder EL. Metabolic epidemiology of colon cancer. Fecal bile acids and neutral sterols in colon cancer patients and patients with adenomatous polyps. Cancer. 1977; 39: 2533-2539.
- Reddy BS. Diet and excretion of bile acids. Cancer Res. 1981; 41: 3766- 3768.
- Payne CM, Bernstein C, Dvorak K, Bernstein H. Hydrophobic bile acids, genomic instability, Darwinian selection, and colon carcinogenesis. Clin Exp Gastroenterol. 2008; 1: 19-47.
- Payne CM, Crowley-Skillicorn C, Bernstein C, Holubec H, Moyer MP, Bernstein H. Hydrophobic bile acid-induced micronuclei formation, mitotic perturbations, and decreases in spindle checkpoint proteins: relevance to genomic instability in colon carcinogenesis. Nutr Cancer. 2010; 62: 825-840.
- Ajouz H, Mukherji D, Shamseddine A. Secondary bile acids: an underrecognized cause of colon cancer. World J Surg Oncol. 2014; 12: 164.
- Ekbom A, Yuen J, Adami HO, McLaughlin JK, Chow WH, Persson I. Cholecystectomy and colorectal cancer. Gastroenterology. 1993. 105: 142- 147.
- Siddiqui AA, Kedika R, Mahgoub A, Patel M, Cipher DJ, Bapat V. A previous cholecystectomy increases the risk of developing advanced adenomas of the colon. South Med J. 2009; 102: 1111-1115.
- Giovannucci E, Colditz GA, Stampfer MJ. A meta-analysis of cholecystectomy and risk of colorectal cancer. Gastroenterology. 1993; 105: 130-141.
- Shao T, Yang YX. Cholecystectomy and the risk of colorectal cancer. Am J Gastroenterol. 2005; 100: 1813-1820.
- Lieberman DA, Holub JL, Moravec MD, Eisen GM, Peters D, Morris CD. Prevalence of colon polyps detected by colonoscopy screening in asymptomatic black and white patients. JAMA. 2008; 300: 1417-1422.
- Ashktorab H, Nouraie M, Hosseinkhah F, Lee E, Rotimi C, Smoot D. A 50-year review of colorectal cancer in African Americans: implications for prevention and treatment. Dig Dis Sci. 2009; 54: 1985-1990.
- Farhana L, Antaki F, Anees MR, Nangia-Makker P, Judd S, Hadden T, et al. Role of cancer stem cells in racial disparity in colorectal cancer. Cancer Med. 2016; 5: 1268-1278.
- Mira-Pascual L, Cabrera-Rubio R, Ocon S, Costales P, Parra A, Suarez A, et al. Microbial mucosal colonic shifts associated with the development of colorectal cancer reveal the presence of different bacterial and archaeal biomarkers. J Gastroenterol. 2015; 50: 167-179.
- Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/ beta-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013; 14: 195-206.
- Nangia-Makker P, Yu Y, Majumdar AP. Role of cancer stem cells in agerelated rise in colorectal cancer. World J Gastrointest Pathophysiol. 2015; 6: 86-89.
- Sanders MA, AP Majumdar. Colon cancer stem cells: implications in carcinogenesis. Front Biosci. 2011; 16: 1651-1662.