Short Communication
Theranostic Applications of Microbubble Sonography in Oncology
Fleischer AC1*, Lyshchik A2 and Caskey C3
1Departments of Radiology and Obstetrics/Gynecology, Vanderbilt University Medical Center, USA
2Department of Radiology, Thomas Jefferson University Hospital, USA
3Vanderbilt Institute of Imaging Science, Vanderbilt University Medical Center, USA
*Corresponding author: Arthur C. Fleischer, Professor, Departments of Radiology & Ob/Gyn, Vanderbilt University Medical Center, 1161 21st Avenue, Nashville, TN 37232-2675, USA
Published: 25 May, 2016
Cite this article as: Fleischer AC, Lyshchik A, Caskey C.
Theranostic Applications of Microbubble
Sonography in Oncology. Clin Oncol.
2016; 1: 1010.
Introduction
For purposes of this short communication, sonographic (ultrasound) techniques that utilize microbubbles (MB) is referred to as microbubble sonography (MBS). MBS has important diagnostic and therapeutic (thus the term "theranostic") clinical applications in targeted or "personalized" oncology. For diagnosis, MBS provides detailed depiction of tumor microvascularity and a means to monitor its response to treatment. Therapeutic use of MB provides a means to enhance drug delivery.
Microbubbles
When used with sonography for diagnostic purposes, microbubbles provide a means to depict the macro- and microscopic (capillary) arrangement of a tumor's vascular network. When performed in 3D, MBS provides volumetric (3D) and dynamic ("real time" 3D or 4D) data. Microbubbles consist of an inert gas encapsulated with a lipoprotein or composite shell (Figure 1). They typically range from 1-7 μ in size, approximately the size of an erythrocyte. MB are injected intravenously and, unlike CT or MRI contrast agents, remain purely intravascular. The shells of MB are eventually metabolized by the liver and the gas portion is eliminated by the lungs 5-7 minutes after injection [1].There have been no reported untoward effects with their use in millions of patients worldwide. In fact, MBS has recently (4/1/16) received FDA approval in the U.S. for focal liver mass and blunt abdominal trauma in children, in addition to their worldwide use in cardiac disorders.
Figure 1
Figure 1
Definity® microbubbles showing variation in sizes from 1-7μ. One of the larger microbubbles in the
center of the picture has ruptured. (c/o Philips Healthcare).
Diagnostic Applications of MBS
Although MBS has been used many years outside the U.S., the recent FDA approval of their use for assessment of liver lesions and in pediatric trauma will undoubtedly result more extensive clinical use. Contrast enhanced ultra sonography (CEUS) using microbubbles have been shown to be accurate in distinguishing benign from malignant hepatic [hepatocellular cancer vs metastases, focal nodular hyperplasia (FNH) vs. hemangioma] and benign vs. neoplastic renal masses, especially in patients with poor renal function who would have contrast related risks for CT and/or MR (Figure 2) [2,3]. Contrast enhancement kinetics allow accurate and early diagnosis in other neoplasms such as those arising within prostate and ovary as documented in many reports including ours based on those patients with a potentially malignant ovarian mass that underwent surgical removal and subsequent pathologic analysis (Figure 3). CEUS coupled with shear wave elastography has been reported to improve detection of prostate cancer even in the presence of multiple central and/or peripheral gland nodules. CEUS has many other uses in renal and hepatic transplants including pseudoaneurysms, hematologically significant stenosis and/or thrombosis in major intraorgan vessels. It is also useful in a variety of vascular disorders such as those associated with arterial and/or venous thrombosis, pseudoaneurysms and arteriovenous fistula. By demonstration of flow within the vaso vasorum of larger vessels such as the carotids, CEUS can be used to detect "vulnerable" atherosclerotic plaque. CEUS is very accurate in detecting liver, splenic and/or renal laceration in patients who have been subjected to blunt abdominal trauma. In the pediatric population, CEUS can be confidently predicted that the use of CEUS will expand due to its lack of ionizing radiation, which is a particular concern in children who typically are subjected to relatively high levels of ionizing radiation with CT. In preclinical and clinical trials, labelled microbubbles can be tailored to depict a variety of targets including neoplastic (VEGF2), inflammatory (p-selectin) a thrombotic processes (α2 β3). This has major applications in preclinical assessment of drug efficacy. As a theranostic agent, once arriving at a particular site, tailored MB may be used to deliver specific medications [4-6] (Figure 5).
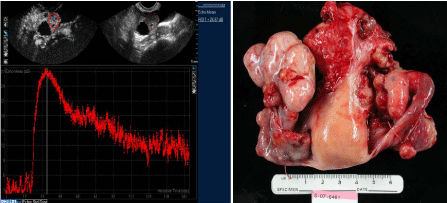
Figure 2
Figure 2
CE-US of stage I ovarian cancer. A. The fundamental image (top right) shows a normal sized ovary
with a 1 cm cystic area. Top left shows a harmonic image of same with the region of interest outlined in red.
The bottom half of the figure shows enhancement kinetic curve with sustained wash-out. Paired contrasted (left)
and fundamental (right) sonograms of hepatocellular carcinoma (Adapted from Fleischer, A., Lyschick, A. JUM
2007). B. Gross pathology showing normal sized right ovary.
Figure 3
Tumor Response
CEUS can show changes in tumor vascularity with effective anti-angiogenic medication in hepatocellular cancer, renal cancer, and cervical cancer as well as after invasive procedures such as transcutaneous arterial chemoembolization (TACE) of hepatocellular cancer [7-9] (Figure 4). Labelled microbubbles can assess the appropriateness of potential treatment regimes in childhood malignancies. Targeted microbubbles can accurately depict the relative efficacy of anti-metabolic and/or anti-angiogenetic medication, as recently reported in a small series of children with refractory metastatic cancer [9].
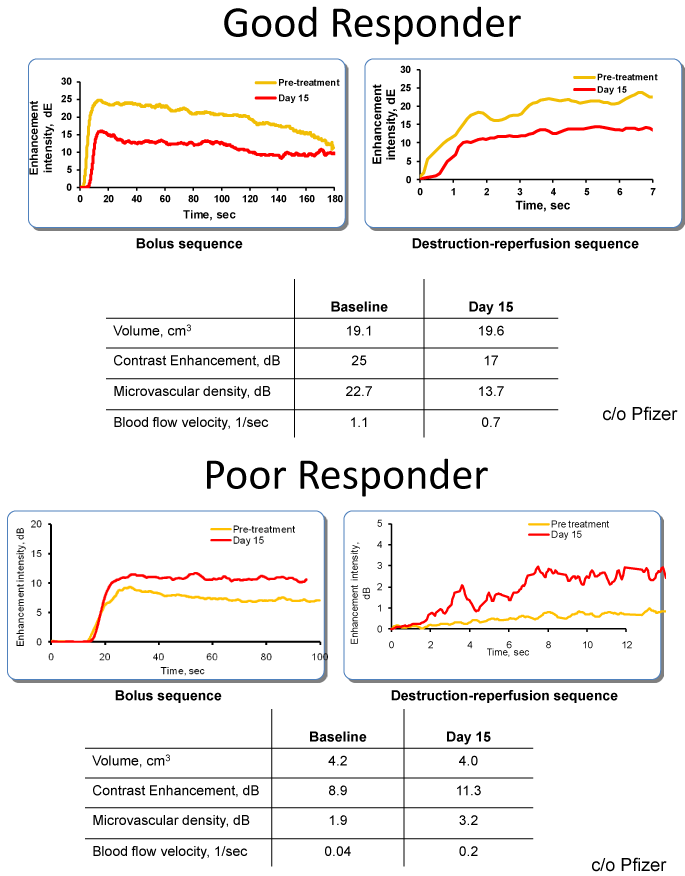
Figure 4
Figure 4
MBS distinguishing between a good vs. poor responder.
1) The post Rx curve (orange) shows reduced perfusion compared to base
line (red). The calculated volume of the lesion was virtually unchanged.
2) Poor responder showing increased perfusion (red) compared to base line
on bolus sequence (left) and destruction reperfusion sequence.
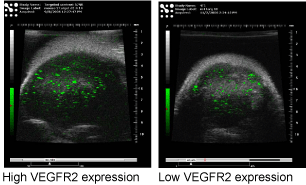
Figure 5
Figure 5
VEGF2targeted tumor showing high (left) vs. low (right) expression
in implanted murine breast tumors.
Therapeutic Applications of MBS
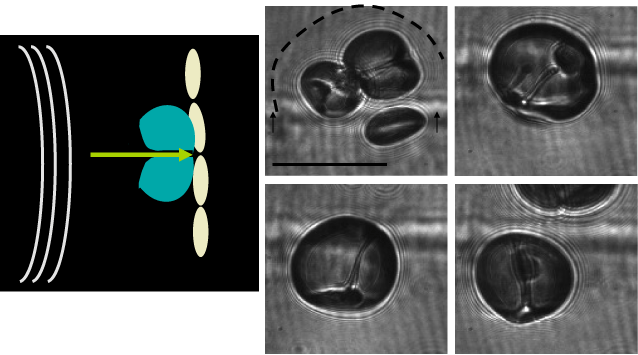
Directed and controlled disruption of microbubbles by increasing the mechanical index of the transmitted ultrasound can focal produce shock waves which, in turn, enhance a medication delivery into the tumor's interstitium across the endothelial gap junction of a vessel's endothelium (Figure 5 and 6). It can also enhance transfection of viruses into tissue. This process is referred to as sonoporation and has been studied in a clinical trial of patients with advanced metastatic pancreatic cancer [10]. Although the number of patients studied is small (n = 10), there appear to be enhanced overall survival of patients with this dreaded neoplasm in a Norwegian study. MBS enhanced sonoporation seemed to improve delivery of chemotherapeutic agents as evidenced by a reduction of tumor size. A recent report from China where High Intensity Focused Ultrasound (HIFU) was used in over 50 subjects with unresectible pancreatic cancer in combination with chemotherapy showed improved survival and amelioration of symptoms in the treated group [11] (Figure 7). Similarly, for nononcologic applications MB have been shown to improve the transfer of antibodies to beta amyloid plaque in the interstitum across the blood brain barrier in a murine Alzheimer's model [12-14].
Figure 6
Figure 6
(left) Diagram of microbubble undergoing cavitation with an arrow
indicating the direction of strong cavitation force impinging on the vascular
endothelium. (right) High-speed microscopy images of microbubbles
undergoing cavitation and producing fluid jets. Caskey, Charles F., et al.
"Microbubble tunneling in gel phantoms". The Journal of the Acoustical
Society of America 125.5 (2009): EL183-EL189.
Future Applications
Future directions of clinical research using MBS includes selective enhanced oxygenation of normally relative hypoxic and therefore relatively refractory radiation therapy. For this, the central gas of the microbubble, typically perfluorocarbon, can be replaced with oxygen. This could improve oxygenation of hypoxic tumors, thus enhancing their response to radiation therapy. Thus it can be confidently predicted that the theranostic applications of MBS will continue to expand into oncology, providing enhanced tumor specificity and therapy. It has already become an important contributor to the new era of targeted, precision medicine and promises to provide more extensive use.
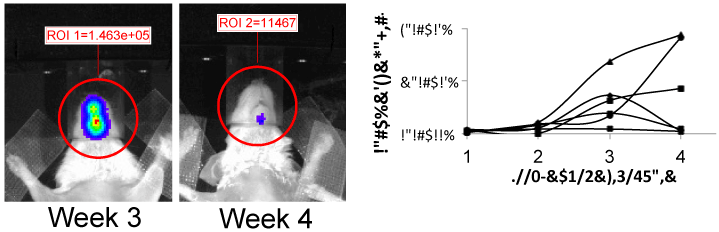
Figure 7
Figure 7
Optical images of murine brain tumor week 3 (left) and 4 (right)
treatment with microbubbles and doxirubin. The graph shows the treated
subjects had better response than controls.
Acknowledgements
This overview is based on several funded grants of the first author including AIUM Discovery Grants (2002, 2004), National Institute of Health 7R21 CA 125227-03 and 1R01DE 024982-01 and Bracco (Milan, Italy) trial# 36636. The author thanks Leona Fleischer for her editorial assistance.
References
- Caissie A, Karshafian R, Hynynen K, Czarnota G. Ultrasound contrast microbubbles: In vivo imaging and potential therapeutic applications. In: Nanoimaging. Goins B, Phillips W, eds. Pan Stanford Pub, 267-291, 2011.
- Wilson SR, Burns PN. Microbubble-enhanced US in Body Imaging: What Role?Radiology. 2010; 257: 24-39.
- Barr RG, Peterson C, Hindi A. Evaluation of indeterminate Renal Masses with Contrast-enhanced US: A Diagnostic Performance Study. Radiology. 2014 April; 271(1): 133-142.
- Fleischer AC, Lyshchik A, Hirari M, Moore RD, Abramson RG, Fishman DA. Early Detection of Ovarian Cancer with Conventional and ContrastEnhanced Transvaginal Sonography: Recent Advances and Potential Improvements. J Oncol. 2012; 2012: 302858.
- Pochon S, Tardy I, Bussat P, Bettinger T, Brochot J, von Wronski M, et al. BR55: A Lipopeptide-based VEGFR2-Targeted Ultrasound Contrast Agent for Molecular Imaging of Angiogenesis. Invest Radiol. 2010 Feb; 45 (2): 89-95.
- Pysz MA, Foygel K, Rosenberg J, Gambhir SS, Schneider M, Willmann JK. Antiangiogenic Cancer Therapy: Monitoring with Molecular US and A Clinically Translatable Contrast Agent (BR55). Radiol. 2010 Aug; 256 (2): 519-527.
- Lassau N, Koscielny S, Chami L, Chebil M, Benatsou B, Roche A, et al. Advanced Hepatocellular Carcinoma: Early Evaluation of Responses to Bevacizumab Therapy at Dynamic Contrast-enhanced US with Quantification – Preliminary Results. Radiol. 2011; 258: 291-300.
- Williams R, Hudson JM, Lloyd BA, Sureshkumar AR, Lueck G, Milot L, et al. Dynamic Microbubble Contrast-enhanced US to Measure Tumor Response to Targeted Therapy: A Proposed Clinical Protocol with Results from Renal Cell Carcinoma Patients Receiving Antiangiogenic Therapy. Radiol. 2011; 260: 581-590.
- McCarville B. CEUS can predict tumor response to drugs in children. St. Jude's Children Hospital Health Care Business 9/14/15.
- Deelman LE, Declèves A-E, Rychak JJ, Kumar S. Targeted Renal Therapies Through Microbubbles and Ultrasound. Advanced Drug Delivery Reviews 2010; 62: 1369-1377.
- Lv W, Yan T, Wang G, Zhao W, Zhang T, Zhou D. High intensity focused ultrasound therapy in combination with gemcitabine for unresectable pancreatic carcinoma. Ther Clin Risk Mgmt 2016: 12: 687-691.
- Gilja OH, Haukeland U. University Hospital, Bergen, Norway. Fiere Drug Delivery 9/15/2015.
- Koh J, Jung DC, Oh YT, Yoo MG, Noh S, Han KH, et al. Additional Targeted Biopsy in Clinically Suspected Prostate Cancer: Prospective Randomized Comparison between Contrast-Enhanced Ultrasound and Sonoelastography Guidance. Ultrasound Med Biol. 2015; 41: 2836-2841.
- . Leinenga, G, Gotz J. Scanning ultrasound removes amyloid-β and restores memory in an Alzheimer's disease mouse model. Sci Transl Med. 2015; 11: 7.