Clinical Image
Input of Next Generation Sequencing for the Diagnosis of an Uncommon Anemia
Meunier M1*, Passet M2, Lefebvre C3, Cahn J-Y1, Burroni B4, Kosmider O2 and Park S1
1University Clinic of Hematology, CHU Grenoble Alpes, TIMC-TheREx (UMR 5525 CNRS, Université Grenoble Alpes), France
2Laboratory of Hematology, Cochin Hospital, Paris Descartes University, France
3Laboratory of Hematology, Onco-genetic and immunology, Biology and Pathology Institute, France
4Department of Pathology, Cochin Hospital, University Hospital Paris-Centre, France
*Corresponding author: Meunier Mathieu, Clinique Universitaire d 'Hématologie, CHU de GRENOBLE CS10217, 38043 Grenoble cedex 09, France
Published: 25 May, 2016
Cite this article as: Meunier M, Passet M, Lefebvre C, Cahn J-Y, Burroni B, Kosmider O, Park S. Input of Next Generation Sequencing for the Diagnosis of an Uncommon Anemia. Clin Oncol. 2016; 1: 1009.
Clinical Image
A 57 year-old man was referred for investigation of anemia in the context of minor betathalassemia
proved by electrophoresis of hemoglobin, dilated cardiomyopathy with aortic ectasia,
asthma, hiatal hernia and cholecystectomy. Anemia was already known due to thalassemia with
hemoglobin level around 100 to 95g/l level, mean corpuscular volume around 58 fl and hemoglobin
electrophoresis with an excess of Hemoglobin A2 and F proving the minor beta thalassemia. But,
since few months, hemoglobin was falling dramatically to 84g/l. A first bone marrow examination
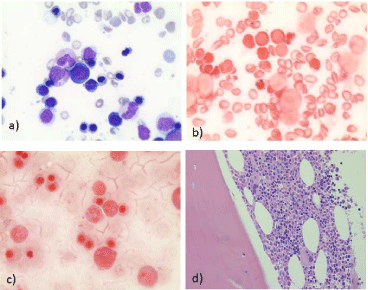
was performed and showed dysplastic features (basophilic stippling, laminated cytoplasm) only on
erythroid lineage (Figure 1A) but involving less than 10% of the lineage, and the presence of 48%
ringed sideroblasts (Figure 1B). Nevertheless those erythroid dysplastic features could be attributed
to thalassemia as well as myelodysplastic syndromes due to the increased erythropoiesis observed in
thalassemia. Ringed sideroblasts are not specific of dysplastic syndrome. Ringed sideroblasts can be
seen in some specific deficiencies (zinc, vitamin B6) [1,2] or intoxications (alcohol, lead, benzene,
isoniazid, copper) [3,4]. All these etiologies were eliminated by questioning and biological blood
tests. EPO level was 20.4 U/l and ferritin level was normal (389ng/ml). The patient received no prior
treatment (neither transfusion, nor erythropoietin stimulating agents (ESA)) and was referred for
a secondary opinion to our university hospital center. At this time, 3 months later after the first
bone marrow examination, a new bone marrow aspiration was performed with a flow cytometry,
karyotype and molecular analyses, and a bone marrow biopsy. Bone marrow aspiration results were
similar to the first one with abnormalities of the erythroid lineage: laminated and granular cytoplasm
and abnormalities of karyorrhexis, but ringed sideroblasts were not found (Figure 1C). Moreover,
the flow cytometry Ogata score was low (score=1/4) [5] and the karyotype were normal. There
were some dystrophic megakaryocytes and micromegakaryocytes in the marrow biopsy without
fibrosis (Figure 1D). At this stage, it was difficult to decide between myelodysplastic syndrome
or increased erythropoiesis due to thalassemia. Finally, the molecular biology analysis using a
next generation of sequencing assay by using the Ion AmpliSeq™ library kit 2 (Life technologies),
found mutations concerning genes that are mostly involved in myelodysplastic syndromes. Deep
sequencing identified 3 candidate somatic mutations: a missense in SETBP1 (I871T) and two ins/del
mutation inducing a premature stop codon in SRSF2 and TET2. All these mutations were previously
described as somatic [6-8] and were confirmed by direct sequencing. Moreover, the variant allele
frequency for each mutation was 45%, 43% and 41% respectively for TET2, SRSF2 and SETBP1,
corresponding probably to one major clone. Concerning the ringed sideroblasts, the SF3B1
mutation which is present in 60-80% of the refractory anemia with ringed sideroblasts (RARS) [9],
was not found in our patient, leading us to think that the ringed sideroblasts were not related to
the typical MDS subtype RARS. Finally, the diagnosis of undetermined myelodysplastic syndrome
(MDS-U) was retained thanks to the presence of an array of arguments: the association of the three
mutated genes in a relatively young patient and with the presence of SRSF2 and SETBP1 mutations,
which are not so commonly found in "control patients without any cytopenia" [10,11], and the
dystrophic megakaryocytes in bone marrow biopsy which are not found in thalassemia. A treatment
with erythropoietin stimulating agents (ESA) was begun. Three months after the beginning of ESA,
Hb level increased from 86g/l to 108g/l which corresponds to a hematological erythroid response
according to IWG 2006 criteria [12].
This case illustrates the aid available from molecular biology analyses in situations where the
diagnosis of myelodysplastic syndrome is not formally raised by
the morphological study of the bone marrow aspiration. Moreover,
in this case, the percentage of morphological abnormalities was
below the percentage required for the diagnosis of myelodysplastic
syndrome and cytogenetic analyses were normal, evoking a new
kind of myelodysplastic syndrome based on a molecular biology
classification [13].
Figure 1
Figure 1
a) May-Grunwald-Giemsa (MGG) stained bone marrow aspiration,
first marrow examination, X400; b) Perls stained bone marrow aspriation,
first marrow examination, X400; c) Perls stained bone marrow aspiration,
second marrow examination, X400; d) Hemalin-Erythrosine-Safran (HES)
stained bone marrow trephine biopsy, X40.
References
- Fiske DN, McCoy HE, Kitchens CS. Zinc-induced sideroblastic anemia: report of a case, review of the literature, and description of the hematologic syndrome. Am J Hematol. 1994; 46: 147-150.
- Haworth C, Evans DI, Mitra J, Wickramasinghe SN. Thiamine responsive anaemia: a study of two further cases. Br J Haematol. 1982; 50: 549-561.
- Gregg XT, Reddy V, Prchal JT. Copper deficiency masquerading as myelodysplastic syndrome. Blood. 2002; 100: 1493-1495.
- Piso RJ, Kriz K, Desax MC. Severe isoniazid related sideroblastic anemia. Hematol Rep 2011; 3: e2.
- Ogata K, Della Porta MG, Malcovati L, Picone C, Yokose N, Matsuda A, et al. Diagnostic utility of flow cytometry in low-grade myelodysplastic syndromes: a prospective validation study. Haematologica, 2009; 94: 1066- 1074.
- Makishima H, Yoshida K, Nguyen N, Przychodzen B, Sanada M, Okuno Y, et al. Somatic SETBP1 mutations in myeloid malignancies. Nat Genet. 2013; 45: 942-946.
- Perez C, Martínez-Calle N, Martín-Subero JI, Segura V, Delabesse E, Fernandez-Mercado M, et al. TET2 mutations are associated with specific 5-methylcytosine and 5-hydroxymethylcytosine profiles in patients with chronic myelomonocytic leukemia. PLoS One. 2012; 7: e31605.
- Wu SJ, Kuo YY, Hou HA, Li LY, Tseng MH, Huang CF, et al. The clinical implication of SRSF2 mutation in patients with myelodysplastic syndrome and its stability during disease evolution. Blood. 2012; 120: 3106-3111.
- Papaemmanuil E, Cazzola M, Boultwood J, Malcovati L, Vyas P, Bowen D, et al. Somatic SF3B1 mutation in myelodysplasia with ring sideroblasts. N Engl J Med. 2011; 365: 1384-1395.
- Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med 2014; 371: 2488-2498.
- Genovese G, Jaiswal S, Ebert BL, McCarroll SA. Clonal hematopoiesis and blood-cancer risk. N Engl J Med, 2015; 372: 1071-1072.
- Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006; 108: 419-425.
- Malcovati L, Papaemmanuil E, Ambaglio I, Elena C, Gallì A, Della Porta MG, et al. Driver somatic mutations identify distinct disease entities within myeloid neoplasms with myelodysplasia. Blood, 2014; 124: 1513-1521.