Research Article
A Dosimetric Comparison between Three Different External Photon Beam Techniques for Accelerated Partial Breast Irradiation
Francesca Bonfantini1, Elena De Martin2, Tommaso Giandini1, Maria Luisa Fumagalli2, Anna Cavallo1, Valentina Pinzi3, Michela Dispinzieri4, Eliana La Rocca4, Riccardo Valdagni4,5, Roberto Agresti6, Laura Fariselli3, Laura Lozza4, Emanuele Pignoli1 and Maria Carmen De Santis4*
1Department of Medical Physics Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, Italy
2Department of Health, Fondazione IRCCS Istituto Neurologico Carlo Besta, Italy
3Department of Neurosurgery Radiotherapy Unit, Fondazione IRCCS Istituto Neurologico Carlo Besta, Italy
4Department of Radiotherapy Unit 1, Fondazione IRCCS Istituto Nazionale dei Tumori, Italy
5Department of Oncology and Hemato-ncology, University degli Studi di Milano, Italy
6Department of Breast Surgery Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy
*Corresponding author: Maria Carmen De Santis, Department of Oncology and Hemato-ncology, University degli Studi di Milano, Radiotherapy Unit 1, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy
Published: 18 Jul, 2018
Cite this article as: Bonfantini F, De Martin E, Giandini T,
Fumagalli ML, Cavallo A, Pinzi V, et
al. A Dosimetric Comparison between
Three Different External Photon Beam
Techniques for Accelerated Partial
Breast Irradiation. Clin Oncol. 2018; 3:
1501.
Abstract
Objectives: To evaluate the advantages and limits of CyberKnife (CK) compared to the two
external beams Radiotherapy (RT) Techniques, Three Dimensional Conformal Therapy and
Volumetric Modulated Arc Therapy(3D-CRT and VMAT) for Accelerated Partial Breast
Irradiation (APBI). A dosimetric study was conducted with special focus on dose to organs at risk
(OAR), target coverage and technical features.
Methods: Ten consecutive early-stage breast cancer patients were selected and for each one of them,
three treatment plans were generated for 3DCRT, VMAT and CK. Dosimetric parameters, extracted
from the dose volume histograms, were used to evaluate the differences in terms of PTV coverage
and OAR sparing among the irradiation techniques. Conformity Index (CI) and Homogeneity
Index (HI) were also compared.
Results: VMAT and CK provided equivalent dose conformity, with CIs significantly higher
compared to 3D-CRT technique. Besides, VMAT achieved the best results in terms of HI and target
coverage (p<0.05). Significant differences were observed in the OAR dosimetric data, except for
heart. 3DCRT achieved the best results in terms of the dose to the whole contra-lateral breast as
regards technical features. The treatment session time is usually longer for CK (on average 60 min)
than for VMAT and 3DCRT techniques (15 to 20 min).
Conclusion: In this dosimetric comparison, all RT techniques are feasible to deliver APBI.CK and
VMAT provide higher conformity than 3D-CRT, although with 3D-CRT we observed a reduction
of the dose to the OAR. In CK treatment organ motion is controlled and, despite the longer
treatment times, the delivery accuracy is expected to be better than 3D-CRT and VMAT, especially
if motion management systems are not used. Advances in Knowledge: 1) CK treatment allows to
reduce safely the PTV margin, achieving both optimal PTV coverage and a better sparing OAR. 2)
This study can provide an important guidance to select the right RT technique for APBI.
Introduction
Breast radiotherapy (RT) after breast-conserving surgery is known to reduce the risk of any breast cancer recurrence by a half and related mortality by a sixth in patients with early breast cancer [1]. While whole-breast RT actually remains the standard of care, consensus statement of the American Society for Radiation Oncology and the European Society for Radiotherapy and Oncology recommended partial breast RT for selected patients at low risk of recurrence because of age, small tumor size and early stage [2,3]. The rationale for investigating partialbreast RT is based on the evidence that the large majority of local recurrences in breast cancer after breast conserving treatment is close to the original tumor site [4,5]. This evidence suggested restricting the RT target to the surgical cavity in selected patients. With a reduced irradiation target volume, patients can tolerate an accelerated regimen of irradiation with an increased daily dose and a significant reduction in overall times. An additional theoretical advantage of accelerated partial breast irradiation (APBI) is a decreased dose to normal tissue. This way, APBI should allow reducing RT morbidity without compromising its ability to cure the cancer. There are a number of approaches now available for the implementation of APBI, i.e.: multi-catheter interstitial brachytherapy [6-9], balloon catheter brachytherapy [10-12], external beam radiation therapy (EBRT) [13-15] and intraoperative radiation therapy [16]. All these techniques show different and peculiar characteristics in terms of degree of invasiveness, radiation delivery, operator proficiency, acceptance between radiation oncologists and length of treatment [17]. Specifically, EBRT has potential advantages over brachytherapy, among which being non-invasive, less operator dependent, and having acceptable cosmetic outcome. The main techniques in use for EBRT are 3- dimensional conformal radiation therapy (3D-CRT) [13,18] and intensity-modulated radiation therapy (IMRT) [19], the latter being frequently delivered as Volumetric Modulated Arc Therapy (VMAT) [20]. CyberKnife (CK) has emerged as a possible alternative to conventional techniques for APBI, although there is still a reduced experience with this technique up to now [21-23]. Since June 2013, a prospective non-randomized trial, designed to assess the toxicity, cosmesis and the feasibility of CK treatments for APBI, started as cooperation between two Institutes in Milan. To evaluate the advantages and limits of CK compared to the two EBRT techniques (3D-CRT and VMAT) normally employed for APBI, a dosimetric study was conducted with special focus on dose to normal breast tissue and (OAR), and on target coverage and technical.
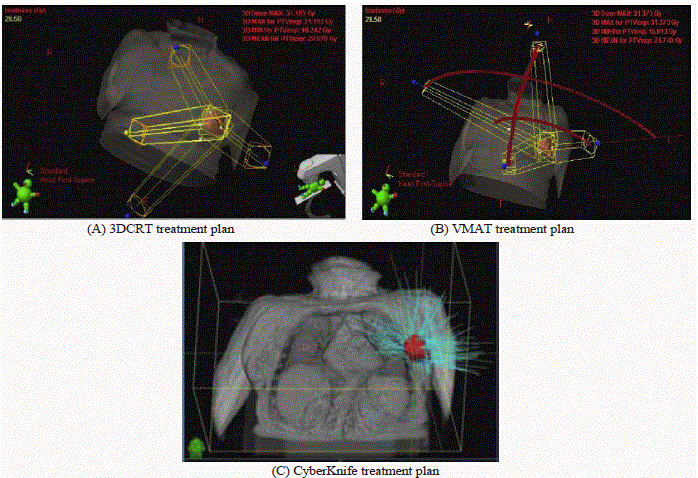
Figure 1
Figure 1
Examples of (A) 3D-CRT with 4 beams (2 coplanar and 2 non-coplanar), (B) VMAT with partial coplanar and non-coplanar arcs, and (C).CK with nonisocentric non-coplanar beam arrangement.
Materials and Methods
Ten consecutive patients with stage I-IIA histologically confirmed
breast carcinoma, with tumorfree inked histologic margins at surgical
resection and enrolled in the clinical trial NCT02896322 [24] were
selected for the study. For each patient, a planning CT scan (1.5 mm
slice thickness) was obtained, from the skull base to the diaphragm,
in supine position with the arms lying along the body to ensure a
comfortable position during CK treatment. Three gold fiducials
were placed in the walls of the surgical cavity at the time of
lumpectomy to allow CK to track respiratory motion. The clinical
target volume (CTV) was defined as tumor bed (GTV, gross target
volume) plus a 15 mm margin to take into account
subclinical disease extension; CTV was limited to 5mm below the
body surface, muscles and chest wall. The planning target volume
(PTV) was obtained as a CTV isotropic expansion of 5 mm to take
into account organ motion and setup errors, clipped 5 mm into
body surface anteriorly and bounded by posterior breast extent.
Heart, bilateral lungs, thyroid, ipsi- and contra-lateral breasts were
separately contoured as OAR according to RTOG guidelines [25].
The non-target breast volume was then obtained by subtracting the
PTV from the ipsi-lateral breast volume. The skin volume was
created with an 3mm contraction of the external body contour
resulting in a shell with 3 mm thickness. The prescription dose (PD)
to the PTV was 30Gy in 5 consecutive daily fractions (6 Gy per
fraction). The planning objectives for PTV coverage and the OAR
dose constraints are summarized in Table 1. Furthermore, hot spots
had to be kept within the PTV and not exceed 115% of PD, whereas
OAR dose-volume constraints should be fulfilled within a 5%
tolerance with respect to the values in Table 1.
Three treatment plans were generated for each patient: 3D-CRT
and VMAT plans were designed using Varian Eclipse (version
11.0.30, Varian Medical Systems, Palo Alto, CA) treatment planning
system (TPS), while CK plans were optimized using Multiplan
(Accuray Incorporated, Sunnyvale, CA) TPS. In particular, 2
coplanar fields plus 2 fields with the treatment couch rotated by 90°
were set for 3D-CRT plans. Two partial coplanar arcs were set for
VMAT: a clockwise gantry rotation from 260-290° to 50° with the
corresponding counterclockwise rotation for right breast treatments,
and from 300-310° to 85-100° for left breast treatments. In the most
challenging cases, two additional arcs were added with the treatment
couch rotated by 90°. Collimator angles were set different from zero
in order to reduce the tongue and groove effect. The dose calculation
algorithm used by Eclipse TPS was the anisotropic analytical
algorithm with a 2-mm calculation grid and heterogeneity
correction. All 3D-CRT and VMAT plans were performed with a 6
MV photon beam produced by a Varian Clinac equipped with a
Millennium Multi Leaf Collimator with 120 leaves.
CK treatment plans were optimized using the variable aperture
Iris collimator to deliver a set of multiple non-isocentric noncoplanar
beams: the entry angles and the total number of beams
were fully managed by the TPS. The dose distribution calculations
were performed using the ray-tracing algorithms with heterogeneity
correction and a high-resolution grid of 1-pixel size. An example of
the beam setup for each of the three RT techniques is shown in Figure
1.
All treatment plans were optimized to achieve optimal PTV
coverage without exceeding OAR constraints. In addition, all
generated plans were acceptable for a treatment delivery.
Treatment plans were evaluated from a technical and dosimetric
point of view. In particular, dosimetric parameters, extracted from
the dose volume histograms (DVHs), were used to evaluate PTV
coverage and OAR sparing for the different irradiation techniques.
For PTV coverage, minimum (Dmin), maximum (Dmax) and
mean dose (Dmean), and the percentage of PTV receiving 90%, 95%
and 105% of the PD (i.e.: V27Gy, V28.5Gy and V31.5Gy, respectively)
were considered.
To evaluate the overall quality of treatment plans, their conformity,
homogeneity, number of monitor units (MU) and delivery treatment
times were also compared. Conformity index (CI) and homogeneity
index (HI) were calculated according to the reported formula [26].
CI=!"!"!"# x !"!"#!"# HI =! !!"!"#.
Where TVPVI is the target volume covered by the prescription
isodose volume, TV is the target volume and PIV is the prescription
isodose volume; Dmax is the maximum point dose and PD is the
prescribed dose to the target volume. CI ranges from 0 to 1, the last
being the ideal case while a value close to 0 indicates a total absence
of conformation [27].
Data were also analyzed dividing the patients into 2 subgroups
based on PTV laterality.
The differences among the three RT techniques were analyzed
by paired Student’s t-test, considering a p-value <0.05 (2-tailed) as
statistically significant. Statistical analysis was performed by using
the MedCalc software (MedCalc® Version 12.1.3.0, MedCalc Software
BVBA 2011, Belgium).
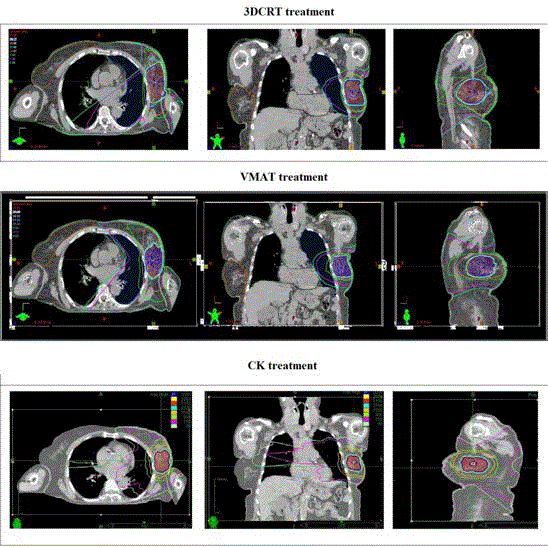
Figure 2
Figure 2
Example of dose distribution obtained with the three techniques for the same left-sided breast cancer patient, in the axial, coronal and sagittal views (red, 31.5 Gy; blue, 30 Gy; yellow, 28.5 Gy; cyan, 27 Gy; orange, 15 Gy; green, 9 Gy; magenta, 1.5 Gy; light green 0.9 Gy).
Results
Five patients received RT to the right breast and five to the left
breast. The tumors were located as follows: 3 in the upper outer
quadrant, 3 in the lower inner quadrant, 2 in the lower outer quadrant
and 2 in the upper inner quadrant of the breast. The average PTV
volume was 121.6 ± 68.2 cc (range: 31.8 – 259.0 cc).
All the planning objectives required by the APBI protocol were
achieved with all techniques.
The results for PTV coverage, CI, HI and the OAR dosimetric
data obtained for each treatment modality are summarized in Table 2.
VMAT and CK provided equivalent dose conformity, with CIs
significantly higher compared to 3D-CRT technique. Besides, VMAT
achieved the best results in terms of HI and target coverage (V27Gy
and V28.5Gy).
An example of representative dose distributions for each
treatment technique is shown in figure 2.
As shown in Table 2, significant differences were observed in
dosimetric data of lungs, thyroid, skin and breast. Concerning heart,
similar dose values were obtained in each right-sided breast cancer
treatments, while low doses were reduced with 3D-CRT compared
to CK and VMAT in left-sided breast cancer treatment. For all
techniques, mean heart dose for right and left-sided breast cancer
were less than 0.5 and 1.1Gy, respectively.
Analyzing the subgroups of patients treated for right and left
breast cancer, the results for PTV coverage proved to be very similar
to those for the whole group of patients, as shown in Table 3. For
both subgroups, the mean percentage of PTV receiving 95% of the
PD (V28.5Gy) was significantly lower for CK compared to 3D-CRT
and VMAT technique, while V31.5Gy and Dmax were significantly
higher in CK plans.
In table 3 was also reported the only significant results for the
OARs.
Comparing right- and left-sided breast treatments for each
RT technique, no significant differences for OARs were observed,
although the doses delivered to thyroid, ipsi-lateral lung and ipsilateral
breast were higher in left breast treatments. Besides, in order
to reduce the heart dose, a small increase of dose to ipsi-lateral lung
and breast was sometimes allowed, slightly increasing the weight of
the beams directed toward these organs.
As to PTV: HI, V31.5Gy and Dmax of VMAT treatments resulted
significantly higher in left-sided than right-sided breast cancer
(p<0.01).
The CK plans, with an average of 122 beams (range: 89-187),
delivered on average approximately 13-18 times more MU over the
course of the treatment than the other two techniques (15229 MU per
fraction for CK vs. 851 MU and 1151 MU per fraction for 3D-CRT
and VMAT, respectively).
The average beam-on time to deliver the 3D-CRT and VMAT
plans was approximately less than 5 and 2 min, respectively. Using
the CyberKnife system with Iris, the treatment time including patient
set-up on treatment couch was approximately 60 min, ranging from
~35 min to ~120 min (beam delivery time: 33.5 ± 9.7 min).
Table 1
Table 2
Table 2
Comparison of PTV and OAR dosimetric data for the three RT approaches. For each variable was reported the mean value ± standard deviation. For each
comparison, only the statistically significant differences are reported (p-values <0.05).
Discussion
The National Surgical Adjuvant Breast and Bowel Project B-06
trial reported that 75% of local recurrences were found at or in
proximity to the lumpectomy cavity [28]. This evidence suggested to
restrict the RT target to the lumpectomy cavity in selected patients
with low recurrence rate risk, using an approach of APBI [2,3].
Moreover, in a context of a modern vision of personalized treatments,
it does not always seem suitable to apply the same radiotherapy to all
the patients.
Multicatheter brachytherapy was the most widely used technique
in APBI and with the largest follow up [9,29-32], although it never
gained wide acceptance because of the complexity and invasiveness of
the procedure and treatment initiation based on the final pathology.
Furthermore, tumor size and location may preclude patients from
receiving APBI with the brachytherapy technique. 3D-CRT offers
a more homogenous dose distribution than brachytherapy-based
APBI does, but could give a higher dose to lung, heart, or the
remaining normal breast. However, the limits of 3D-CRT concern
dosimetry, motion, and cosmesis. Usually, a larger margin is used
with this technique, potentially increasing toxicity and decreasing
cosmesis outcomes. Two recent reports investigating 3D-CRT using
conventional linear accelerators for APBI have raised concerns for
unacceptable cosmesis [14,33]. The authors illustrated that in patients
developing unacceptable cosmesis, the mean volume of breast
receiving 50% and 100% of the prescribed dose was significantly
higher than in patients with acceptable cosmesis [33].
Several studies have investigated the use of 3D-CRT and the
modern RT techniques, including IMRT [15,34], VMAT [20] and
CyberKnife [21-23,35] for APBI. Recently we have published our
results, in terms of acute/subacute toxicity, of a pilot study for APBI
by CK. In particular we showed as, by using CK and a fractionation of
30 Gy in 5 fractions, very good cosmetic results were achievable [24].
In this study, we performed a technical and dosimetric comparison
among 3D-CRT, VMAT and CK for an APBI clinical protocol using
10 patients treated by CK as reported in the study of Lozza et al. [24].
We evaluated the plans considering different dosimetric
parameters for the PTV, such as target coverage, conformity and
homogeneity indexes, and the doses delivered to the OARs, and
technical aspects of dose delivery, such as the total number of MUs
and the treatment delivery time. All the planning objectives required
by the APBI protocol were achieved by all techniques.
VMAT and CK provided equivalent dose conformity, significantly
better than 3D-CRT, with a consequent better sparing of the non-
PTV ipsi-lateral breast. Besides, VMAT achieved the best results in
terms of HI and percentage of target volume receiving 90% of PD.
Our results confirm those reported by Qiu et al., who
demonstrated that VMAT can improve dose conformity and reduce
the beam delivery time, as compared with 3D-CRT.
Similarly, Heinzerling et al. [36] found that the CK treatment
planning for PBI allows achieving very conformal target coverage
while significantly reducing dose to OARs, as ipsi-lateral lung and
heart, compared to 3D-CRT. Also, Goggin et al. [37] showed that
CK offers both a higher conformity than 3D-CRT due to the higher
number of non-coplanar beams, and a less normal breast tissue
exposure attributable to image-guided tracking.
The maximum doses and HIs of PTV for our CK plans are higher
than the other two techniques: these results are expected considering
the inherent property of CK to create heterogeneous dose distributions
inside the PTV. This explains also the significantly higher values of
V27Gy and V28.5Gy obtained with3D-CRT and VMAT.
Significant differences were observed in the OAR dosimetric data,
except for heart. However, low doses to heart were in general reduced
with 3D-CRT in left-sided breast patients and this is expected to
reduce the risk of radiation-related cardiac disease.
Lung doses were significantly lower for 3D-CRT than for VMAT.
This result is opposed to what obtained by Qui et al. [20]. This
discrepancy is probably due to the different planning approach: Qui
et al. optimized VMAT plans using specific avoidance sectors to avoid
entry angles directed toward the lungs and heart, thus reducing their
absorbed dose.
3DCRT achieved the best results in terms of the dose to the whole
contra-lateral breast thanks to the beam arrangement; however, the
mean doses were very low for all three techniques.
The localization (position and depth) and shape of the PTV and its
relative position with respect to the OARs can significantly influence
the treatment plan optimization and, consequently, the treatment
results [38-40]. Moreover, the planning CT scans used in this study
were acquired in supine position with the arms lying along the body
to ensure a comfortable position during the long lasting treatments
performed with CK. This setup necessarily limits the available
degrees of freedom for the beam angles and the arc rotations in the
3DCRT and VMAT planning, respectively. In particular, to avoid
arm irradiation and to keep the dose to the heart as low as possible
a small increase in the dose to the ipsi-lateral lung and the contralateral
breast is expected. The patient position should be choice in
an appropriate manner to ensure a certain comfort in order to get
a reproducible and stable position during irradiation. Anyway, all
the 3DCRT and VMAT treatment plans fully fulfilled the dosimetric
objectives, proving that this kind of patient setup can be managed
with a proper treatment plan optimization.
The treatment session time is usually longer for CK (on average
60 min) than for VMAT and 3DCRT techniques (15 to 20 min
dependently if non-coplanar beams are used), both because of longer
time needs for dose delivery and for patient setup and fiducials
alignment phase. In general, larger breasts were associated with
an increased mobility, requiring longer patient set-up times with
CK. Furthermore, CK delivered on average approximately 13-18
times more MU over the course of the treatment than the other two
techniques, which may lead to increased total body scattered dose, as
described by Hermando et al. [41] and Vallis et al. [42].
However, VMAT minor delivery time potentially reduces the
inaccuracy that maybe caused by respiratory motion or errors
in patient setup, although in many cases the use of a respiratory
management system is recommended if a proper expansion of PTV
margin is used. Different studies [43-45] showed the efficiency both
of 3D-CRT and VMAT delivery using breath-hold techniques or
respiratory-gating systems for APBI. In fact, in EBRT difficulties in
set up reproducibility and organ motion result in larger expansion
margins to make up for target localization uncertainties [46-47].
Unfortunately, this larger margin can result in greater normal
breast tissue volume receiving high dose irradiation. Furthermore,
even by using wider margins the interplay effect (interplay between
respiration-induced tumor motion and the dynamic dose delivery)
can be not negligible in VMAT treatment, especially if delivered in
hypo fractionated regime [48-49].
Between the three RT modalities analyzed in this study, the
CyberKnife offers meaningful technical improvements to existing PBI
techniques using real time tracking, respiratory motion management
with Synchrony system [50] and sub-millimeter accuracy. This could
allow reducing the margins of PTV in CK treatment, minimizing the
doses at the OARs without compromising the target coverage.
Conclusion
In this dosimetric comparison, all RT techniques are feasible
to delivery APBI.CK and VMAT provide higher conformity than
3D-CRT, although with 3D-CRTwe observed a reduction of the dose
to the OARs except ipsi-lateral breast.
In CK treatment organ motion is controlled and, despite the
longer treatment times, the delivery accuracy is expected to be better
than 3D-CRT and VMAT, especially if motion management systems
are not used. The results of this study can provide certain guidance
for clinicians that want to apply a clinical protocol for ABPI taking
into account the specific technologies available in their own hospital.
Clinical considerations about the impact of the dosimetric difference
on patients’ toxicity are necessary to select the optimal RT technique
for each patient treated with APBI.
Acknowledgment
This study was partially funded by the LILT Lega Italiana per la Lotta contro i Tumori (Milano).
References
- Darby S, Mc Gale P, Correa C, Taylor C, Arriagada R, Clarke M, et al. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis on individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707-16.
- Smith DB, Arthur DW, Buchholz TA, Haffty BG, Hahn CA, Hardenbergh PH, et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO). Int J Radiat Oncol Biol Phys. 2009;74(4):987-1001.
- Polgár C, Van Limbergen E, Pötter R, Kovács G, Polo A, Lyczek J, et al. GEC-ESTRO breast cancer working group.Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: Recommendations of the Groupe Européen de CuriethérapieEuropean Society for Therapeutic Radiology and Oncology (GEC-ESTRO) breast cancer working group based on clinical evidence. Radiother Oncol. 2009. 94(3):264-273.
- Mannino M, Yarnold JR. Local relapse rates are falling after breast conserving surgery and systemic therapy for early breast cancer: can radiotherapy ever be safely withheld? Radiother Oncol. 2009;90(1):14-22.
- Salvadori B, Marubini E, Miceli R, Conti AR, Cusumano F, Veronesi U, et al. Reoperation for locally recurrent breast cancer in patients previously treated with conservative surgery. Br J Surg. 1999;86(1):84-7.
- Perera F, Engel J, Holliday R, Scott L, Girotti M, Girvan D, et al. Local resection and brachytherapy confined to the lumpectomy site for early breast cancer: a pilot study. J Surg Oncol. 1997;65(4):263-7.
- Baglan KL, Martinez AA, Frazier RC, Kini VR, Kestin LL, Chen PY, et al. The use of highdose-rate brachytherapy alone after lumpectomy in patients with early-stage breast cancer treated with breast-conserving therapy. Int J Radiat Oncol Biol Phys. 2001;50(4):1003-11.
- Polgar C, Major T, Fodor J, Sulvok Z, Somogyi A, Lovey K, et al. Accelerated partial-breast irradiation using high-dose-rate interstitial brachytherapy: 12-year update of a prospective clinical study. Radiother Oncol. 2010;94(3): 274-9.
- Aristei C, Maranzano E, Lancellotta V, Chirico L, Zucchetti C, Italiani M, et al. Partial breast irradiation with interstitial multi-catheter high-doserate brachytherapy. Long-term results of a phase II prospective study. Radiotherm Oncol. 2017;124(2):208-213.
- Keisch M, Vicini F, Kuske RR, Hebert M, White J, Quiet C, et al. Initial clinical experience with the MammoSite breast brachytherapy applicator in women with early-stage breast cancer treated with breast-conserving therapy. Int J Radiat Oncol Biol Phys. 2003,55:289-93.
- Bensaleh S, Bezak E, Borg M. Review of MammoSite brachytherapy: advantages, disadvantages and clinical outcomes. Acta Oncol. 2009;48(4):487-94.
- Vicini F, Beitsch P, Quiet C, Gittle-man M, Zannis V, Fine R, et al. Fiveyear analysis of treatment efficacy and cosmesis by the American Society of Breast Surgeons MammoSite Breast Brachytherapy Registry Trial in patients treated with accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2010;79(3):808-17.
- Baglan KL, Sharpe MB, Jaffray D, Frazier RC, Fayad J, Kestin LL, et al. Accelerated partial breast irradiation using 3D conformal radiation therapy (3D-CRT). Int J Radiat Oncol Biol Phys. 2003;55(2):302-11.
- Hepel JT, Tokita M, Macausland SG, Evans SB, Hiatt JR, Price LL, et al. Toxicity of Three- Dimensional Conformal Radiotherapy for Accelerated Partial Breast Irradiation. Int J Radiat Oncol Biol Phys. 2009;75(5):1290-6.
- Meattini I, Saieva C, Miccinesi G, Desideri I, Francolini G, Livi L, et al. Accelerated partial breast irradiation using intensity modulated radiotherapy versus whole breast irradiation: Health-related quality of life final analysis from the Florence phase 3 trial. Eur J Cancer. 2017;76:17-26.
- Veronesi U, Orecchia R, Luini A, Galimberti V, Zurrida S, Intra M, et al. Intraoperative radiotherapy during breast conserving surgery: a study on 1822 cases treated with electrons. Breast Cancer Res Treat. 2010;124(1):141-51.
- Goyal S, Khan AJ, Haffty BG Advances in radiotherapy for breast cancer. In: Antoinette R. Tan, guest editor. Breast Cancer, Demos Medical Publishing. 2010:487.
- Coles CE, Griffin CL, Kirby A, Titley J, Agrawal R, Alhasso A, et al. Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial. The Lancet. 2017;390(10099):1048-60.
- Livi L, Buonamici FB, Simontacchi G, Scotti V, Fambrini M, Compagnucci A, et al. Accelerated partial breast irradiation with IMRT: new technical approach and interim analysis of acute toxicity in a phase III randomized clinical trial. Int J Radiat Oncol Biol Phys. 2010;77(2):509-15.
- Qiu JJ, Chang Z, Wu QJ, Yoo S, Horton J, Yin FF. Impact of volumetric modulated arctherapy technique on treatment with partial breast irradiation. Int J Radiat Oncol Biol Phys. 2010; 78(1):288-296.
- Rahimi A, Thomas K, Spangler A, Rao R, Leitch M, Wooldridge R, et al. Preliminary Results of a Phase 1 Dose-Escalation Trial for Early-Stage Breast Cancer Using 5-Fraction Stereotactic Body Radiation Therapy for Partial-Breast Irradiation. Int J Radiat Oncol Biol Phys. 2017;98(1):196- 205.
- Bondiau PY, Bahadoran P, Lallement M, Birtwisle-Peyrottes I, Chapellier C, Chamorey E, et al. Robotic stereotactic radioablation concomitant with neo-adjuvant chemotherapy for breast tumors. Int J Radiat Oncol Biol Phys. 2009;75(4):1041-1047.
- Vermeulen S, Haas JA. Cyber Knife stereotactic body radiotherapy and Cyber Knife accelerated partial breastirradiation for the treatment of early breast cancer, Transl Cancer Res. 2014;3(2):295-302.
- Lozza L, Fariselli L, Sandri M, Rampa M, Pinzi V, De Santis MC. Partial breast irradiation with Cyberknife after breast conserving surgery: a pilot study in early breast cancer. Radiation Oncology. 2018;13(1):49.
- Julia White, An Tai, Douglas Arthur, Thomas Buchholz, Shannon MacDonald, Lawrence Marks, et al. Breast Cancer Atlas for Radiation Therapy Planning: Consensus Definitions. RTOG.
- Paddick I. A simple scoring ratio to index the conformity of radiosurgical treatment plans. Technical note J Neurosurg. 2000;93(3):219-22.
- Feuvret L, Noel G, Mazeron JJ, Bey P. Conformity Index: a Rewiew. Int J Radiation Oncology Biol Phys. 2006; 64(2):333–342.
- Fisher ER, Anderson S, Tan-Chiu E, Fischer B, Eaton L, Wolmark N. Fifteen-year prognostic discriminants for invasive breast carcinoma: National Surgical Adjuvant Breast and Bowel Project Protocol -06. Cancer. 2001; 91(8):1679-87.
- Arthur DW, Vicini FA. Accelerated partial breast irradiation as a part of breast conservation therapy. J Clin Oncol. 2005;23(8):1726-35
- Goyal S, Kearney T, Haffty BG. Current application and research directions for partial-breast irradiation. Oncology (Williston Park). 2007;21(4):449- 61.
- Offersen BV, Overgaard M, Kroman N, Overgaard J. Accelerated partial breast irradiation as part of breast conserving therapy of early breast carcinoma: a systematic review. Radiother Oncol. 2009;90(1):1-13.
- Polgar C, Major T, Fodor J, Sulyok Z, Somogyi A, Lövey K, et al. Accelerated partial-breast irradiation using high-dose-rate interstitial brachytherapy: 12-year update of a prospective clinical study. Radiother Oncol. 2010;94:274-79.
- Jagsi R, Ben-David M, Moran J, Marsh RB, Griffith KA, Hayman J, Aet al. Unacceptable cosmesis in a protocol minvestigating intensity-modulated radiotherapy with active breathing control for accelerated partial-breast irradiation. Int J Radiat Oncol Biol Phys. 2009;76(1):71-8.
- Moran JM, Ben-David MA, Marsh RB, Balter JM, Griffith KA, Hayman JA, et al. Accelerated partial breast irradiation: What is dosimetric effect of advanced technology approaches? Int J Radiat Oncol Biol Phys. 2009;75(1):294-301.
- Rault E, Lacornerie T, Dang H-P, Crop F, Lartigau E, Reynaert N, et al. Accelerated partial breast irradiation using robotic radiotherapy: a dosimetric comparison with tomotherapy and three-dimensional conformal radiotherapy. Radiat Oncol. 2016;11:29.
- Heinzerling JH, Ding C, Ramirez E, Chang K, Anderson JF, Edwards CM, et al. Comparative dose-volume analysis for CyberKnife and 3D conformal partial breast irradiation treatment of early stage breast cancer. Int J Radiat Oncol Biol Phys. 2010;78(3):S825-6.
- Goggin LM, Descovich M, McGuinness C, Shiao S, Pouliot J, Park C. Dosimetric comparison between 3-Dimensional conformal and robotic SBRT treatment plans for accelerated partial breast radiotherapy, Technology in Cancer Research & Treatment. 2016;15(3):437-45.
- Toscas JI, Linero D, Rubio I, Hidalgo A, Arnalte R, Escudé L, et al. Boosting the tumorbed from deep-seated tumors in early-stage breast cancer: A planning study between electron, photon, and proton beams. Radiother Oncol. 2010;96(2):192-8.
- Bouchardy C, Rapiti E, Usel M, Balmer Majno S, Vlastos G, Benhamou S, et al. Excess ofcardiovascular mortality among node-negative breast cancer patients irradiated for innerquadrant tumors. Ann. Oncol. 2010;21:459-65.
- Kim MJ, Park SH, Son SH, Cheon KS, Choi BO, Suh TS. Comparison study of the partial breast irradiation techniques: dosimetric analysis of threedimensional conformal radiation therapy, electron beam therapy, and helical tomotherapy depending on various tumor locations. Med Dosim. 2013;38(3):327-31.
- Hermando ML, Marks LB, Bentel GC, Zhou SM, Hollis D, Das SK, et al. Radiation-induced pulmonary toxicity:adose-volumehistogramanalysisin 201patientswithlungcancer. Int J Radiat Oncol Biol Phys. 2001;51(3):650-9.
- Vallis KA, Pintilie M, Chong N, Holowaty E, Douglas PS, Kirkbride P, et al. Assessment ofcoronary heart disease morbidity and mortality after radiation therapy for early breast cancer. J Clin Oncol. 2002;20(4):1036-42.
- Essers M, Osman S, Hol S, Donkers T, Poortmans PM. Accelerated partial breast irradiation (APBI): Are breath-hold and volumetric radiation therapy techniques useful? Acta Oncologica. 2014; 53(6):788-94.
- Moran JM, Ben-David MA, Marsh RB, Balter JM, Griffith KA, Hayman JA, et al. Accelerated partial breast irradiation: What is dosimetric effect of advanced technology approaches? Int J Radiat Oncol Biol Phys. 2009;75(1):294-301.
- Nicolini G, Vanetti E, Clivio A, Fogliata A, Cozzi L. Pre-clinical evaluation of respiratorygated delivery of volumetric modulated arc therapy with RapidArc. Phys Med Biol. 2010;55(12):N347-57.
- Cox BW, Horst KC, Thornton S, Dirbas FM. Impact of increasing margin around the lumpectomy cavity to define the planning target volume for 3D conformal external beam Accelerated partial breast irradiation. Med Dosim. 2007;32(4):254-62.
- Hasan Y, Kim L, Wloch J, Chi Y, Liang J, Martinez A, et al. Comparison of planned versus actual dose delivered for external beam accelerated partial breast irradiation using cone-beam CT and deformable registration. Int J Radiat Oncol Biol Phys. 2011;80(5):1473-6.
- Yu CX, Jaffray DA, Wong JW. The effects of intra-fraction organ motion on the delivery of dynamic intensity modulation. Phys Med Biol. 1998;43(1):91-104.
- Bortfeld T, Jiang SB, Rietzel E. Effects of motion on the total dose distribution. Semin Radiat Oncol. 2004;14(1):41-51.
- Pepin EW, Wu H, Zhang Y, Lord B. Correlation and prediction uncertainties in the cyber knife synchrony respiratory tracking system. Med Phys. 2011;38(7):4036-44.