Review Article
Surgical Margins in Head and Neck Cancer: Intra- and Postoperative Considerations
Robbins KT1*, Triantafyllou A2, Suárez C3,4, López F5, Hunt JL6, Strojan P7, Williams MD8, Braakhuis BJM9, De Bree R10, Hinni ML11, Kowalski LP12, Rinaldo A13, Rodrigo JP5, Poorten VV14, Nixon IJ15, Takes RP16, Silver CE17 and Ferlito A18
1Division of Otolaryngology-Head and Neck Surgery, Southern Illinois University School of Medicine, USA
2Department of Dentistry, University of Liverpool, UK
3Department of Dentistry, Institute of Health Research of the Principality of Asturias, Spain
4Department of oncology, University of Oviedo, Spain
5Department of Otolaryngology, University of Oviedo, Spain
6Department of Pathology, University of Arkansas for Medical Sciences, USA
7Department of Radiation Oncology, Institute of Oncology, Slovenia
8Department of Pathology, The University of Texas MD Anderson Cancer Center, USA
9Department of Otolaryngology- Head and Neck Surgery, VU University Medical Center, Netherlands
10Department of Head and Neck Surgical Oncology, University Medical Center Utrecht, Netherlands
11Department of Otolaryngology-Head and Neck Surgery, Mayo Clinic, USA
12Department Otolaryngology -Head and Neck Surgery, Centro de Tratamento e Pesquisa Hospital do Cancer A.C. Camargo, Brazil
13Department Otolaryngology -Head and Neck Surgery , University of Udine School of Medicine, Udine, Italy
14Department of Otolaryngology -Head and Neck Surgery and Oncology, University Hospitals Leuven, Belgium
15Departments of Surgery and Otolaryngology, Head and Neck Surgery, Edinburgh University, UK
16Department of Otolaryngology-Head and Neck Surgery, Radboud University Medical Center, Netherlands
17Department of Surgery, University of Arizona College of Medicine, USA
18International Head and Neck Scientific Group, Italy
*Corresponding author: Murat Durdu, Department of Dermatology, Başkent University Faculty of Medicine, Adana Hospital, Seyhan, 01250, Adana, Turkey
Published: 08 Jul, 2018
Cite this article as: Durdu M, Koçer NE. Persistent
Napkin Dermatitis: Langerhans Cell
Histiocytosis. Clin Oncol. 2018; 3: 1493.
Abstract
Objective: To provide a perspective on the significance as applied to current practice, we analyzed
recent reports on optimizing cancer free surgical margins that have challenged standard practices
and novel techniques to assess for occult cancer cells.
Method: We conducted a review of the recent literature (2012-2018) using the keywords surgical
margin analysis, frozen and paraffin section techniques, oral cancer, and head and neck cancer.
Results: Of significance are the reports indicating superiority of tumor specimen directed sampling
of margins compared to patient directed (tumor bed) sampling for frozen section control of oral
cancers. With reference to optimal distance between tumor and the surgical margin, recent reports
recommended cutoffs less than 5 mm. Employment of new technologies such as light spectroscopy
and molecular analysis of tissues, provide opportunities for a “real time” assessment of surgical
margins.
Conclusion: The commonly practiced method of patient directed margin sampling involving
previous studies raises concern over conclusions made regarding the efficacy of frozen section
margin control. The recent studies that challenge the optimal distance for clear surgical margins
are retrospective and address patient cohorts with inherently confounding factors. The use of novel
ancillary techniques requires further refinements, clinical trial validation, and justification based on
the additional resources.
Keywords: Surgical margins; Techniques; Frozen sections; Spectroscopy; Molecular markers
Background
Cancer surgery aims towards achieving complete resection of
the tumor without leaving behind residual disease. Intra-operative
assessment of margins includes inspection (open or video assisted),
palpation, and the use of histopathologically examined frozen sections.
Post-operatively, the definitive assessment of margins is established
via histopathologic examination of the resection specimen.
In the management of head and neck cancer, achieving adequate
surgical margins devoid of cancer cells is influenced by anatomical
sub-site, biologic behavior, proximity to important structures,
expected functional impairment, quality of life and esthetic
compromise, and previous treatment. Recurrences managed by
surgery present additional challenges as microscopic tumor elements
may have spread below normal mucosa and at a distance from the
original site, which adversely affects determination of margins [1,2].
Appreciating growth patterns of particular head and neck cancers,
and the effects of anatomical sub-sites influence the surgeon when
removing the tumor while minimizing the risk for leaving residual
disease. These factors are further examined in turn:
a) Growth patterns: These are broadly classified as “pushing” or
“irregular and infiltrative”, including the extension along planes of
least resistance or formation of satellite lesions. The role of formation
of desmoplastic stroma in tumor growth and progression [3] and
“field cancerization” should also be considered. Finally, attention
should be given to adjacent nerves and vessels; select head and neck
malignancies such as adenoid cystic carcinoma may spread along
nerves.
b) Site of Origin: For cancers of the oral tongue, floor of mouth and
buccal mucosa, the depth of invasion into the underlying musculature
is important. Satisfactory peripheral (mucosal) margins around the
superficial component of those tumors may be easier to achieve in
comparison to a deep, intramuscular component. The perimysium of
musculature may be a pathway of least resistance for tumor spread
and the surgeon should adapt the excision to compensate for this.
Cancers arising in the larynx, hypopharynx, oropharynx, and
nasopharynx, present additional surgical challenges. For example,
submucosal extension is well recognized for hypopharyngeal
carcinoma whereas removing nasopharyngeal cancer is influenced
by its anatomical proximity to vital structures. In addition, the
increasing popularity of minimally invasive surgery has challenged
the concept of wide surgical margins for laryngeal and oropharyngeal
carcinoma [4].
As regards cancers of the sinonasal cavities, resection with wide
margins is not always possible, as this may affect cranial nerves, orbit,
internal carotid artery, or brain. Attempts to remove the tumor with
wide surgical margins could therefore result in undesirable morbidity
and would be technically difficult, if not impossible [5].
Frozen Section-Based Analysis of Surgical Margins
The use of frozen section margins is influenced by institutional
setting and available resources. Furthermore, there is wide variation
among surgeons and pathologists on the extent of margin evaluation
at the time of surgery [6]. In the United States, frozen section analysis
is practiced widely for patients undergoing extirpative procedures for
upper aerodigestive tract carcinomas. In particular, it has become of
greater importance in minimally invasive procedures.
Surgical centers constructed to incorporate a frozen section
facility within the operating suite, make it feasible for the surgeon
to walk the specimen into this facility and directly converse with
the pathologist. This has the advantage of optimizing specimen
orientation and enabling consensus on sampling. An in-situ frozen
section facility seems indispensable when multiple specimens are
removed in a tangential (parallel to the mucosal surface) plane.
Surgeons adopt these strategies when sampling strips of tissue
from the tumor bed, which may harbor residual disease; and for
minimal access procedures when the tumor is resected in multiple
parts. The latter may be adversely influenced by site. For example,
lymphoid tissue surrounding the base of tongue may be clinically
misinterpreted as tumor whereas tumor invasion of the intrinsic
tongue base musculature may become obscured by retraction of the
severed muscle bundles.
Frozen section facilities that are within the surgical suites are not
features of many surgical centers. This probably influences national,
multidisciplinary management guidelines for head and neck cancer.
For instance, an influential publication explicitly states that “intraoperative
frozen sections have a limited role in patient management”
and that “frozen sections are appropriately used for the assessment of
surgical excision margins when there is clinical doubt as to adequacy”
[7]. “Appropriate use” is influenced by the experience of the surgeon,
site and size of the tumor and distance between operating theater and
pathology laboratory, in turn. An experienced surgeon would likely
achieve satisfactory, mucosal and deep margins for T1-2 tumors of
the tongue or buccal mucosa; accordingly, frozen sections may not be
necessary. It also may be technically difficult to achieve satisfactory
margins for T3-4 tumors in difficult anatomical locations and frozen
section in this setting may offer limited further information. A further
factor to be considered in the analysis of the surgical specimen is the
type of information expected. Often, only a “positive” or “negative”
characterization is enough. In this case, the surgeon samples
suspicious areas of the tumor bed/surroundings. Less frequently,
information on a particular edge of the submitted specimen is
requested. In this case the specimen may be submitted with a marker
suture, or an accompanying diagram/short description defining that
edge. Here, the pathologist orientates the specimen as above, but also
applies ink or dyes to the edge or makes a diagram of the mounted
specimen profile to indicate it.
There is a paucity of studies that have analyzed the treatment
efficacy for frozen section techniques. DiNardo et al. examined the
frozen section results, permanent controls, and final tumor margins
from 80 consecutive patients [8]. Tumor location was varied including
oral cavity in 23 (28.75%), oropharynx in 22 (27.50%), larynx in 17
(21.25%), hypopharynx in 9 (11.25%), sinonasal region in 5 (6.25%),
and major salivary gland in 4 (5.00%). Note should be made that all
intra-operative frozen sections were taken from the surgical bed. The
overall accuracy of frozen section in the evaluation of close or positive
final margins was 71.3% (sensitivity, 34.3%; specificity, 100%). In
addition only 4 of 80 of patients (5%) potentially benefited from intraoperative
frozen section by virtue of immediate margin revision. The
estimated cost of intra-operative frozen section averaged as much as
$3,123 per patient, with a cost-benefit ratio of 20:1. They concluded
that intra-operative frozen section margins are accurate yet costly and
unreliable for eradicating positive final margins.
A survey among head and neck surgeons in the American Head
and Neck Society indicated there was no agreement on surgical
margin assessment as well as defining a clear surgical margin. Seventysix
percent of surgeons stated that they only sampled the tumor bed
to determine intra-operative margin status [9].
In another report, Black et al surveyed 200 pathologists in North
America about their center's current process of frozen-section
margin evaluation [10]. The responders indicated that the majority
of surgeons send small fragments of tissue from the surgical defect.
Many pathologists receive small slices of specimens that were not
oriented. Many pathologists resample all or most of the margins
for the final pathology report without anatomic orientation from
the surgeon. Other pathologists do not sample any margins. The
results indicated a lack of a standard method of margin analysis for
this practice. Also there was difficulty in addressing frozen section
versus final section margin discrepancies after the specimen had been
completely sectioned underscoring the need for interdisciplinary
communication early in the process. The responses indicated a lack
of communication for orientation of the tissue received for frozen
section, thus compromising the accuracy of the final pathology
report. Respondents who expressed the most satisfaction with their
systems were those that had early and regular communication with
the surgeons. The report concluded that consensus was lacking on
how to best submit tissue for frozen-section evaluation of head and
neck resection margins.
A more recent Canadian study analyzed the impact of frozen
section assessment of operative margins on survival in 416 surgically
treated oral cancer patients [11]. 229 patients who had frozen
sections were compared by univariate and multivariate analysis with
197 patients who did not have frozen sections. Again, when frozen
sections were performed, the additional margins were taken from
the patient. The results showed that failure at the primary site was
independently influenced by age at diagnosis (P .001), T stage (P
.016), N stage (P .042), and status of margins on paraffin sections
(P .005). The chance of achieving clear margins on paraffin sections
was not significantly improved by the use of frozen sections. They
concluded that the use of frozen sections did not independently have
an impact on local failure or survival.
In a very recent publication supporting the use of frozen section
analysis, Mayfield et al. studied compared 1796 pairs of frozen section
and corresponding permanent sections: positive versus negative [12].
Discordances were found only in 55 (3.1%) pairs. They concluded
that frozen section is an accurate method for evaluation of operative
margins for head and neck carcinomas with concordance between
frozen and permanent results of 97%. Most errors are false negative
results with the majority of these being due to sampling issues. Note
should be made that the tissues submitted for frozen section analysis
were selected and sent separately by the surgeon. In most cases, the
face of the specimen representing the true operative margin was not
designated by the surgeon. The specimens taken from the patient
were submitted entirely for frozen section evaluation and sampled
from the surface designated as representing the “true new margin”
when so identified. The tissue site of origin was documented for each
specimen, among which oral cavity comprised approximately one
half.
Review of the Technique for Harvesting Tissue for Frozen Section Analysis
Surprisingly, there has been very little attention given to
identifying which sampling technique offers the most accurate
approach for determining surgical margin status, both in terms of
frozen sections and paraffin embedded tissue. A couple of recent
studies suggest that samples taken for frozen sections from the excised
cancer (tumor directed sampling) for analysis of margins are more
accurate than samples taken from the tumor bed (patient directed
sampling), at least for oral cancer [13]. Vaveres et al. retrospectively
evaluated 108 patients who underwent surgery for oral cancer. Frozen
section was performed with the surgeon and pathologist agreeing
where on the specimen the frozen sections should be taken. Ninetyone
patients (84.3%) had frozen sections taken from the specimen,
eight from the tumor bed, and nine had none taken at the time of
surgery. The overall local recurrence rate was 18.5%, 25% in patients
who had margins taken from the tumor bed and 17.6% when taken
from the specimen.
Amit et al further investigated this [14] in a prospective,
randomized controlled trial comparing 2 methods of intra-operative
margin assessment: specimen-driven margins and patient-driven
margins. The analysis included 71 patients, of whom 20 (29%) were
in the patient- driven margin arm. Frozen section analysis revealed
positive/close surgical margins that led to an extension of the surgical
resection in 22 of 51 patients (43%) in the specimen-driven margin
arm, and 2 of 20 patients (10%) in the patient-driven margin arm.
Final pathological analysis showed a wide negative margin rate of
84% in the specimen- driven margin arm, compared to 55% in the
patient-driven margin arm (p=02). Extension of the surgical resection
prevented escalation of adjuvant treatment in 19 patients (38%) in the
specimen-driven margin arm and 10% in the patient-driven margin
arm.
It is reasonable to conclude that patient driven harvesting of tissue
margins, particularly from the deep surfaces of the tumor bed, is more
fraught with error when identifying possible residual cancer cells. This
has particular relevance when assessing margins following the en bloc
excision of larger (T2-4) oral cancers in which the surgeon often faces
a tumor bed that is 2-3 fold larger than the excised specimen owing to
retraction of the deep soft tissue. An additional concern is that patient
directed sampling underestimates the real margin status given that
close margins will be categorized as negative as the separate margins
are evaluated ‘en face’ and cannot directly measure the distance from
the tumor front to the margin. Furthermore, pathologists are more
likely to detect small nests of tumor from margins taken from the
specimen closest to the palpable core of the tumor.
Bone margin is a limitation of frozen section analysis because it
is not feasible to evaluate an un-decalcified specimen. Bilodeau and
Chiosea [15] reported the alternative use of intra-operative bone
marrow curettings and inferior alveolar nerve biopsies as a surrogate
of bone margin assessment in patients submitted to mandibulectomy.
The method showed a 50% sensitivity and 100% specificity.
Definition of a Clear Margin
The post-operative histopathologic assessment of cancer
specimens for tumor involvement of the surgical margins is a requisite
for head and neck cancer treatment. Depending on the distance
between the front of the invasive tumor and peripheral (mucosal)
or deep margins, the National Comprehensive Cancer Network
(NCCN) defines a clear margin (negative) as >5.0 mm; close (1-5
mm); and involved (positive) (<1.0mm) (Figure 1). In 2005, Woolgar
and Triantafyllou histopathologically appraised the surgical margins
in 301 resection specimens of oral and oropharyngeal squamous cell
carcinoma [16]. Those with involved margins (23%) were influenced
by anatomical factors, pT and histological parameters of the tumor
(eg, growth pattern). With regard to outcome, [17] Dillon et al.
Reported that oral cancer patients with clear surgical margins showed
higher disease-free survival rates than those with close or involved
margins. Similarly, Yamada et al showed that a margin within 1, 2,
or 4 mm respectively of the tumor edge, all had a proportionately
increased risk of local recurrence, which was significantly different
from oral cancer patients with margins >5 mm [18].
Recent publications have stirred a debate as to how much distance
from invasive tumor represents a clear margin. Dik et al. Analyzed
200 stage I-II oral cancers with 2 or less unfavorable histological
parameters (spidery infiltrative, peri-neural and vascular-invasive
growth) and reported that survival of patients with margins of 3 mm
or more was as good as patients with margins of 5 mm or more [19].
However, the use of additional treatment for patients with close or
positive margins (re-resection - 31 patients; post-operative radiation
therapy 39 patients) may represent a confounding factor in this
series. Zenoni et al. also challenged the 5.0 mm consensus, advocating
that a mere 3 mm margin may be sufficient [20]. This retrospective
study involved head and neck pathologists who reviewed archival
specimens from 381 patients with oral cancer subjected to primary
surgical resection. Among the patients included in the analysis, the
optimal margin associated with Locoregional Free Survival (LRFS)
was determined to be 2.2 mm. Patients with a margin of 2.3 to 5.0
mm had similar LRFS as patients with a margin of greater than 5.0
mm, and the Hazard Ratio [HR] was only 1.31, whereas the HR for
positive margin was 9.03 and the HR for 0.01- to 2.2-mm margin,
2.83. Again, the use of adjuvant treatment in 95 patients (24.9%),
including RT alone in 65 (17.1%) and chemotherapy and RT in 30
(7.9%) may represent a confounding factor. Again, when interpreting
these results, one should be cognizant of the difficulty in interpreting
measurements archival tissue specimens in which tissue shrinkage
and prior frozen sections may have altered the distances. Tasche et
al. also analyzed the association between local recurrence rates and
distance from invasive tumor to surgical margin in 432 patients with
oral cancer [21]. They found a local recurrence rate of 28% for margins
less than 1 mm and 17% for 1 mm. For margins less than 2 mm, 3
mm, 4 mm, and 5mm, the differences were less varied. In this series,
all patients had frozen sections taken from the tumor bed rather than
the specimen, again raising the concern for underestimating the true
distance of the surgical margins and potential inaccuracies of patient
directed sampling.
Contrary to the definition of adequate margins for oral cancer, an
adequate margin of excision for vocal cord cancers has been accepted
as much less, usually greater than 1 mm. Alicandri-Ciufelli et al.
performed a qualitative meta-analysis to determine what constituted
a close margin and reported that it was ≤ 1 mm in glottic tumors,
whereas greater in other sites (supraglottis ≤ 5 mm; oral cavity ≤ 4
mm; oropharynx ≤ 5 mm) [22].
With regard to midline visceral cancers of the head and neck,
recent reports of what constitutes an adequate margin are influenced
by the development of minimal access techniques and differences in
the biologic behavior. For example, close margins within the larynx
may have a lesser negative impact on disease control [23].
Finally, an important caveat to recognize when assessing the
status of surgical margins is the shrinkage of tissues during routine
processing for histopathologic examination. Umstattd et al. [24]
found a pre-excision to post-fixation mean decrease in tumor-free
margin measurements of 11.3% (95% CI 2.9-19.6%, p=0.011). Most
of this decrease occurs prior to fixation, and may be due to intrinsic
tissue properties rather than the effect of the fixative. El-Fol et al.
[25] in 61 patients with oral cancer who underwent resection of the
tumor with a measured clinical margin of 1.0 cm, compared intraoperative
margins with their histopathologic counterparts; the mean
discrepancy was 47.6% for the buccal mucosa, 4.8% for the floor of
mouth, 9.5% for the mandibular alveolus, 4.8% for the retromolar
trigone, and 33.3% for the tongue. This suggests that shrinkage
is greater for specimens removed from the oral tongue and buccal
mucosa hence, a more liberal macroscopic margin during the excision
is advisable.
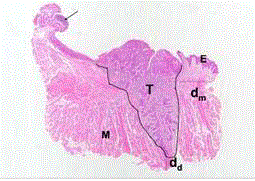
Figure 1
Figure 1
Routine histological section of a resected, squamous cell carcinoma
of the lateral border of tongue. The curved line indicates the silhouette of the
Tumor (T). The horizontal (dm) and vertical (dd) lines indicate the distance of
the advancing front of the tumor from the nearest mucosal and deep resection
margins, respectively. While the mucosal margin appears free, tumor is close
to the deep margin. E: lingual surface epithelium; M: musculature. The arrow
indicates the lingual tonsil. Modified from Woolgar and Triantafyllou (2005).
Recent Ancillary Techniques
Advances in light spectroscopy, endoscopy, imaging, biochemical
alterations of tissue, molecular markers and epigenetic alterations,
allow applications in assessing the status of surgical margins.
Spectroscopy-related techniques
The refinement of spectroscopy techniques eventually may allow
surgeons to practice “real time” enhanced visualization of occult nests
of cancer cells within apparent normal mucosa surfaces surrounding
the tumor. Toward this goal, Francisco et al compared the surgical
margins of oral squamous cell carcinoma to the mucosa of healthy
volunteers using the technique of fluorescent spectroscopy [26]. They
observed discrimination between normal mucosa, injury (presumably
trauma) and surgical margins, demonstrating qualitative differences
in the obtained spectra. Taking this a step further, Poh et al. tested
Fluorescence Visualization (FV)-guided vs. conventional surgery in
156 oral squamous cell carcinomas of less than 4 cm surface diameter
and 90 high-grade pre-invasive lesions (severe dysplasia, carcinoma
in situ) [27]. In the squamous cell carcinoma group, 92 patients in the
FV subset showed significant reduction in the 3-year local recurrence
rate (40.6% vs. 6.5%, p<0.001); among the pre-invasive lesions, 62
patients in the FV group showed a reduction in local recurrence
rate (39.3% vs. 8.1%, p<0.001). Whereas the efficacy of fluorescent
technology appears positive, the logistics of its use as a routine
technique remains to be proven. Unfortunately this technique only
examines the mucosal lining while the deep margin is more frequently
the place where negative margins are difficult to obtain.
The automated and biocompatible handheld mass spectrometry
device (named MasSpec Pen), which rapidly identifies the molecular
profile of tissues using a small volume water droplet, has also been
recently considered [28]. After 3 seconds of gentle physical contact
with a tissue surface, the droplet is transported to a mass spectrometer
that characterizes diagnostic proteins, lipid, and metabolites. The
MasSpec Pen was used to distinguish tumor from healthy tissue
during surgery performed in tumor-bearing mouse models, without
requiring specific labeling or imaging and without tissue destruction.
The authors regarded it as allowing cancer prediction with a high
sensitivity (96.4%), specificity (96.2%) and overall accuracy (96.3%).
However, this technology remains pre-clinical and awaits application
to human trials.
Raman spectroscopy may enable discrimination between tumor
and surrounding healthy tissues in samples from oral cancer patients
and thus is used for “real time” objective intra-operative evaluation
of surgical margins [29]. This technology, based on observing
vibrational, rotational and other low-frequency modes in a system, is
often used in chemistry to provide a structural fingerprint by which
molecules can be identified. It relies on inelastic (Raman) scattering
of monochromatic light, usually from a laser, which interacts with
molecular vibrations or other excitations in the system, effecting
up or down shifting in the energy of the laser photons. Again, this
technology remains in its infancy in terms of clinical application.
Another technique that may be helpful in allowing “real
time” assessment of tumor resection margins intraoperatively is
Cerenkov Luminescence Imaging (CLI) [30]. Positron-Emission
Tomography (PET) radiopharmaceuticals emit optical photons
via Cerenkov luminescence. Cerenkov photons are generated by
positrons traveling faster than the phase velocity of light in tissue,
have a continuous energy spectrum, and can be detected using optical
imaging. CLI purportedly combines high diagnostic performance and
clinical translatability of PET imaging with high spatial resolution
and compactness of optical cameras. Successful intra-operative
assessment of tumor resection margins using CLI after intravenous
injection of 2-deoxy-2-(18F) fluoro-D-glucose has been reported
in breast-conserving surgery [25]. Applications in head and neck
cancers are awaited.
Endoscopic approaches
Miles et al. correlated high-resolution microendoscopy images
obtained during surgery with histopathologic diagnosis to determine
their ability to differentiate between benign and malignant mucosa in
“real time” [31]. The mean accuracy, sensitivity and specificity were
95.1%, 96% and 95%, respectively; the negative predictive value was
98%, and positive predictive value was 91%.
Other strategies have included Narrow Band Imaging (NBI).
This high-resolution endoscopic technology may assist in identifying
potentially neoplastic changes of the epithelium. Unlike conventional
white-light endoscopy, NBI utilizes two distinct wavelengths of light,
415 nm (blue) and 540 nm (green), with bandwidths of 20 nm to 30
nm each, limiting penetrance of the light to the mucosal surface and
improving visualisation of vessels therein. Sifrer et al. in a prospective
study evaluated 45 patients subjected to the intra-operative assessment
of margins by NBI; the control group included 55 patients who had
only undergone standard assessment of margins through frozen
sections [32]. All patients underwent resection of the tumor and
frozen section assessment of peripheral margins. The rate of initial
complete resection in the study group and in the control group was
88.9% and 70.9% (p=0.047), whereas the ratio of histopathologically
negative margins was 95.9% and 88.4%, respectively (p = 0.017). Plaat
et al. examined patients who underwent transoral laser surgery for
early glottic cancer using white light with or without NBI [33]. Local
recurrences developed in 14% of the 93 cases: 12 of 51 patients (24%)
were treated by transorla laser surgery on whilte light alone, and in 1
of 42 patients (2%) in the NBI group (P <0.01). Two-year recurrencefree
survival was 82% in the white light only group and 98% in the
NBI group (P <0.05). Similar findings were reported by Garofolo et al.
in early glottic carcinoma [34]. A limitation of this technique is that it
is only useful for peripheral (mucosal) margins.
Imaging techniques
Optical Coherence Tomography (OCT) is an emerging
technology for performing high-resolution cross-sectional imaging.
It is analogous to ultrasound imaging, except that it uses light instead
of sound. OCT can provide cross-sectional images of tissue structure
on the micron scale in situ and in “real time”. Hamdoon et al. tested it
in the assessment of oral squamous cell carcinoma resection margins
[35]. They observed a mean epithelial thickness of 360 μm and 567
μm at free and tumor-involved margins, respectively. Accordingly,
tumor-involved margins may be identified by architectural changes
and an increased epithelial thickness on OCT images. However,
confirmation of results is desirable and the technology is only useful
for assessment of the peripheral margins.
Regular ultrasound can also be used to assist surgical resections
in oral cancer. Baek et al. reported on 20 patients with clinical T1-2
tongue cancers which were removed by intraoral sonographyassisted
resection [36].The deep safety margins were more adequate
for intraoral sonography-assisted resection (9.8 +/- 5.2 mm) than for
T stage-matched resections without intraoral sonography-assisted
resection (4.0 +/- 2.03 mm) (P<0.001), while the mucosal safety
margins were not different.
Experimental image guided surgical techniques include the use
of fluorescence-labeled monoclonal antibodies and a near infrared
camera and ultrasound [37]. Rapid analysis methods for intraoperative
assessment of bone resection margins by immunoblotting
or intra-operative cell isolation in patients with head and neck cancer
have also been developed [38].
Molecular approaches
The cancer progression models explaining how cancer develops
may also explain local recurrences in patients with histopathologically
negative margins [39]. Braakhuis et al. analyzed DNA patterns
between index primary head and neck cancers and relapses [40].
The latter were accordingly classified as: a) true recurrent tumors
(sharing the same genetic alterations with the index) attributable
to incomplete initial resection; b) second field tumors (sharing
some genetic alterations with the index) unrelated to involvement
of margins and attributable to further genetic “hits”; c) true second
primaries showing significantly different genetic alterations. In
at least 25% of the surgically treated head and neck squamous
cell carcinoma patients, genetic alterations can be detected in the
surgical margins [41]. Therefore, it appears worthy of pursuing
methodologies testing whether un-resected areas are more likely to
develop a local recurrence, and identifying molecular risk factors that
predict malignant transformation therein. Intra-operative molecular
analysis of surgical margins should be carefully balanced against time,
logistics and more routine factors such as site and size of the tumor
[42].
The presence of genetically altered cells can be detected in
histologically normal mucosal margins with methodologies assessing
TP53 mutations, Loss Of Heterozygosity (LOH), Eukaryotic
Translation Initiation Factor 4E (eIF4E), proto-oncogene over
expression and promoter hypermethylation.
Following the pioneering work of Brennan et al, who correlated
the mutational status of p53 in histopathologically negative surgical
margins with the risk of local recurrence, other authors investigated
prediction of relapse in tumor-free tumor margins [43,44]. In a
prospective study of 76 patients with histopathologically tumor-free
margins, van Houten et al. determined the presence of TP53-mutated
DNA in the surgical margins using the phage plaque assay and
correlated it with the clinical outcome [45]. In 20% of the cases, the
presence of TP53-mutated DNA in the surgical margins was found to
be related to the presence of tumor-related precursor (pre-malignant)
lesions.
According to Graveland et al. LOH at 9p and p53 immunostaining
have the most predictive potential, which increases with the
combination of LOH at 9p and/or a large p53 positive field [41].
Singh et al investigated a series of 24 head and neck cancer patients
with negative surgical margins, and reported that while 3/7 (42.8%)
patients with recurrent tumor had p53 positive margins, 6 (85.7%)
of those patients had eIF4e positive margins [46]. Overexpression of
eIF4E may thus be a more accurate prognostic marker compared with
overexpression of p53.
The expression of the PTHLH (Parathyroid Hormone Like
Hormone) EPCAM (Epithelial Cellular Adhesion Molecule), LGALS1
(Galectin 1), MET (proto-oncogene, receptor tyrosine kinase) genes
has been examined for the detection of molecular alterations in
histolopathologically negative surgical margins in 55 patients with
head and neck squamous cell carcinoma by means of real time
quantitative polymerase chain reaction (RTqPCR) [47]. Although
38% of the patients were margin-positive for overexpression of at
least one of those genes, only Metalloproteinase (MMP9) and PTHLH
were associated with the development of second primary tumors and
lower rates of local control, respectively.
Promoter hypermethylation has been studied in cancer-free
surgical margins of head and neck. Supic et al. found that the specific
promoter hypermethylation of DAPK (Death Associated Protein
Kinase) detected in surgical margins, may be a useful molecular
marker for poor survival in oral squamous cell carcinoma patients
[48]. The methylated gene combination of EDNRB and HOXA9 in
margin imprints was the most powerful predictor of poor recurrencefree
survival [48]. Roh et al. used the same method for p16, DCC,
KIF1A, and EDNRB, and compared levels of methylation in head and
neck carcinomas per se and deep margins [49]. Hypermethylation
was detected in 12/17 tumors and in 8 deep margins; during followup,
recurrence occurred in 6 cases of which 5 had molecularly positive
margins. On the other hand, promoter methylation of p16INK4A,
cytoglobin, E-cadherin and TMEFF2 did not add any prognostic
information to histopathologic reporting of surgical margins in oral
squamous cell carcinoma [50].
PAX5 methylated imprint margins were an excellent predictor of
poor LRFS (HR, 3.89; 95% Confidence Interval (CI), 1.19-17.52; P =
0.023) by multivariate analysis. PAX5 methylation appears to be an
excellent tumor-specific marker for molecular deep surgical margin
analysis of HNSCC. To re-emphasize, the molecular assessment of
surgical margins is currently investigational. A significant drawback
is the considerable time required to perform such an assessment,
which limits its intra-operative usefulness.
Conclusion
While it remains clear that the presence of a surgical margin devoid of cancer cells has a major influence on treatment outcome, there is controversy on issues related to tumor free margin distance and techniques for intra-operative margin assessment. The traditional standard of 5 mm distance between the tumor edge and the surgical margin appears invalid for laryngeal cancer and is being challenged for other pharyngeal sites resected by minimal access techniques. Furthermore, recent reports suggest that distances less than 5 mm may also suffice for oral cancers. However, caution is advised before accepting changes in the current standards until additional studies are done, which are designed to minimize confounding factors. For oral cancer, the commonly practiced technique of sampling the tumor bed (patient directed) to determine the status of surgical margins, has been challenged by two recent studies that indicate specimen directed sampling is superior. Additional prospective comparison trials are needed to confirm this apparent difference. However, an additional implication of these results is that previous studies reporting the efficacy of frozen section analysis based on patient directed sampling may not be representative. Finally, assessment of novel technologies for margin assessment, particularly light spectroscopy and molecular analysis of tissues, indicates that opportunities for a “real time” assessment of surgical margins are possible. However, further refinements and validation are needed before it becomes applicable as a standard of care.
References
- Zafereo ME, Hanasono MM, Rosenthal DI, Sturgis EM, Lewin JS, Roberts DB, et al. The role of salvage surgery in patients with recurrent squamous cell carcinoma of the oropharynx. Cancer. 2009;115(24):5723-33.
- Ho AS, Kraus DH, Ganly I, Lee NY, Shah JP, Morris LG. Decision making in the management of recurrent head and neck cancer. Head Neck. 2014;36(1):144-51.
- Afzal S, Lalani EN, Poulsom R, Stubbs A, Rowlinson G, Sato H, et al. MMT-MMP and MMP-2 mRNA expression in human ovarian tumors: possible implications for the role of desmoplastic fibroblasts. Hum Pathol. 1998;29(2):155-65.
- Hinni ML, Ferlito A, Brandwein-Gensler MS, Takes RP, Silver CE, Westra WH, et al. Surgical margins in head and neck cancer: a contemporary review. Head Neck. 2013;35(9):1362-70.
- Llorente JL, López F, Suárez C, Hermsen MA. Sinonasal carcinoma: clinical, pathological, genetic and therapeutic advances. Nat Rev Clin Oncol. 2014;11(8):460-72.
- Baumeister P, Baumüller K, Harréus U, Reiter M, Welz C. Evaluation of margins in head and neck squamous cell carcinoma from the surgeon's perspective. Head Neck. 2018;40(5):963-72.
- Helliwell TR, Woolgar JA, Giles TE, Simpson RHW, Stephenson TJ. Chapter 13. Pathological Assessment. In: Head and Neck Cancer: Multidisciplinary Management Guidelines, 4th Ed. British Association o Otorhinolaryngology Head and Neck Surgery. The Royal College of Surgeons of England, London, 2011, pp 83-93.
- DiNardo LJ, Lin J, Karageorge LS, Powers CN. Accuracy, utility, and cost of frozen section margins in head and neck cancer surgery. Laryngoscope. 2000;110(10 Pt 1):1773-6.
- Meier JD, Oliver DA, Varvares MA. Surgical margin determination in head and neck oncology: current clinical practice. The results of an International American Head and Neck Society Member Survey. Head Neck. 2005;27(11):952-8.
- Black C, Marotti J, Zarovnaya E, Paydarfar J. Critical evaluation of frozen section margins in head and neck cancer resections. Cancer. 2006;107(12):2792-800.
- Pathak KA, Nason RW, Penner C, Viallet NR, Sutherland D, Kerr PD. Impact of use of frozen section assessment of operative margins on survival in oral cancer. Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(2):235-9.
- Layfield EM, Schmidt RL, Esebua M, Layfield LJ. Layfield Frozen Section Evaluation of Margin Status in Primary Squamous Cell Carcinomas of the Head and Neck: A Correlation Study of Frozen Section and Final Diagnoses. Head Neck Pathol. 2018;12(2): 175-80.
- Vavares MA, Poti S, Kenyon B, Christopher K, Walker RJ. Surgical margins and primary site resection in achieving local control in oral cancer resections. Laryngoscope. 2015;125(10):2298-307.
- Amit M, Na'ara S, Leider-Trejo L, Akrish S, Cohen JT, Billan S, et al. Improving the rate of negative margins after surgery for oral cavity squamous cell carcinoma: A prospective randomized controlled study. Head Neck. 2016;38 Suppl 1:E1803-9.
- Bilodeau EA, Chiosea S. Oral squalmous cell carcinoma with mandibular bone invasion: intraoperative evaluation of bone margins by routine frozen secection. Head Neck Pathol. 2011;5(3):216-20.
- Woolgar JA, Triantafyllou A. A histopathological araisal of surgical margins in oral and oropharyngeal cancer resection specimens. Oral Oncol. 2005;41(10):1034-43.
- Dillon JK, Brown CB, McDonald TM, Ludwig DC, Clark PJ, Leroux BG, et al. How does the close surgical margin impact recurrence and survival when treating oral squamous cell carcinoma? J Oral Maxillofac Surg. 2015;73(6):1182-8.
- Yamada S, Kurita H, Shimane T, Kamata T, Uehara S, Tanaka H, et al. Estimation of the width of free margin with a significant impact on local recurrence in surgical resection of oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2016;45(2):147-52.
- Dik EA, Willems SM, Ipenburg NA, Adriaansens SO, Rosenberg AJ, van Es RJ. Resection of early oral squamous cell carcinoma with positive or close margins: relevance of adjuvant treatment in relation to local recurrence: margins of 3 mm as safe as 5 mm. Oral Oncol. 2014;50(6):611-5.
- Zanoni DK, Migliacci JC, Xu B, Katabi N, Montero PH, Ganly I, et al. A Proposal to Redefine Close Surgical Margins in Squamous Cell Carcinoma of the Oral Tongue. JAMA Otolaryngol Head Neck Surg. 2017;143(6):555-60.
- Tasche KK, Buchakjian MR, Pagedar NA, Sperry SM. Definition of "Close Margin" in Oral Cancer Surgery and Association of Margin Distance With Local Recurrence Rate. JAMA Otolaryngol Head Neck Surg. 2017;143(12):1166-72.
- Alicandri-Ciufelli M, Bonali M, Piccinini A, Marra L, Ghidini A, Cunsolo EM, et al. Surgical margins in head and neck squamous cell carcinoma: what is 'close'? Eur Arch Otorhinolaryngol. 2013;270(10):2603-9.
- Nakayama M, Holsinger C, Okamoto M, Seino Y, Miyamoto S, Takeda M, et al. Clinicopathological analyses of fifty supracricoid laryngectomized specimens: evidence base supporting minimal margins. ORL J Otorhinolaryngol Relat Spec. 2009;71(6):305-11.
- Umstattd LA, Mills JC, Critchlow WA, Renner GJ, Zitsch RP 3rd. Shrinkage in oral squamous cell carcinoma: An analysis of tumor and margin measurements in vivo, post-resection, and post-formalin fixation. Am J Otolaryngol. 2017;38(6):660-2.
- El-Fol HA, Noman SA, Beheiri MG, Khalil AM, Kamel MM. Significance of post-resection tissue shrinkage on surgical margins of oral squamous cell carcinoma. J Craniomaxillofac Surg. 2015;43(4):475-82.
- Francisco AL, Correr WR, Azevedo LH, Kern VG, Pinto CA, Ko-walski LP, et al. Fluorescence spectroscopy for the detection of potentially malignant disorders and squamous cell carcinoma of the oral cavity. Photodiagnosis Photodyn Ther. 2014;11(2):82-90.
- Poh CF, Anderson DW, Durham JS, Chen J, Berean KW, MacAulay CE, et al. Fluorescence Visualization-Guided Surgery for Early-Stage Oral Cancer. JAMA Otolaryngol Head Neck Surg. 2016;142(12):209-16.
- Zhang J, Rector J, Lin JQ, Young JH, Sans M, Katta N, et al. Nondestructive tissue analysis for ex vivo and in vivo cancer diagnosis using a handheld mass spectrometry system. Sci Transl Med. 2017;9(406):3968.
- Cals FL, Bakker Schut TC, Hardillo JA, Baatenburg de Jong RJ, Koljenović S, Puppels GJ. Investigation of the potential of Raman spectroscopy for oral cancer detection in surgical margins. Lab Invest. 2015;95(10):1186-96.
- Grootendorst MR, Cariati M, Pinder SE, Kothari A, Douek M, Ko-vacs T, et al. Intraoperative Assessment of Tumor Resection Margins in BreastConserving Surgery Using 18F-FDG Cerenkov Luminescence Imaging: A First-in-Human Feasibility Study. J Nucl Med. 2017;58(6):891-8.
- Miles BA, Patsias A, Quang T, Polydorides AD, Richards-Kortum R, Sikora AG. Operative margin control with high-resolution optical microendoscopy for head and neck squamous cell carcinoma. Laryngoscope. 2015;125(10):2308-16.
- Šifrer R, Urbančič J, Strojan P, Aničin A, Žargi M. The assessment of mucosal surgical margins in head and neck cancer surgery with narrow band imaging. Laryngoscope. 2016;127(7):1577-82.
- Plaat BEC, Zwakenberg MA, van Zwol JG, Wedman J, van der Laan BFAM, Halmos GB, et al. Narrow-band imaging in transoral laser surgery for early glottic cancer in relation to clinical outcome. Head Neck. 2017;39(7):1343-8.
- Garofolo S, Piazza C, Del Bon F, Mangili S, Guastini L, Mora F, et al. Intraoperative narrow band imaging better delineates superficial resection margins during transoral laser microsurgery for early glottic cancer. Ann Otol Rhinol Laryngol. 2015;124(4):294-8.
- Hamdoon Z, Jerjes W, McKenzie G, Jay A, Hopper C. Optical co-herence tomography in the assessment of oral squamous cell carcinoma resection margins. Photodiagnosis Photodyn Ther. 2016;13:211-7.
- Baek CH, Son YI, Jeong HS, Chung MK, Park KN, Ko YH, et al. Intraoral sonography-assisted resection of T1-2 tongue cancer for adequate deep resection. Otolaryngol Head Neck Surg. 2008;139(6):805-10.
- Rosenthal EL, Warram JM, de Boer E, Chung TK, Korb ML, BrandweinGensler M, et al. Safety and Tumor Specificity of Cetuximab-IRDye800 for Surgical Navigation in Head and Neck Cancer. Clin Cancer Res. 2015;21(16):3658-66.
- Nieberler M, Häußler P, Kesting MR, Kolk A, Stimmer H, Nentwig K, et al. Intraoperative cell isolation for a cytological assessment of bone resection margins in patients with head and neck cancer. Br J Oral Maxillofac Surg. 2017;55(5):510-6.
- Cabanillas R, Llorente JL. The Stem Cell Network model: clinical implications in cancer. Eur Arch Otorhinolaryngol. 2009;266(2):161-70.
- Braakhuis BJ, Bloemena E, Leemans CR, Brakenhoff RH. Molecular analysis of surgical margins in head and neck cancer: more than a marginal issue. Oral Oncol. 2010;46(7):485-91.
- Graveland AP, Golusinski PJ, Buijze M, Douma R, Sons N, Kuik DJ, et al. Loss of heterozygosity at 9p and p53 immunopositivity in surgical margins predict local relapse in head and neck squamous cell carcinoma. Int J Cancer. 2011;128(8):1852-9.
- Hayashi M, Wu G, Roh JL, Chang X, Li X, Ahn J, et al. Correlation of gene methylation in surgical margin imprints with locoregional recurrence in head and neck squamous cell carcinoma. Cancer. 2015;121(12):1957-65.
- Brennan JA, Mao L, Hruban RH, Boyle JO, Eby YJ, Koch WM, et al. Molecular assessment of histopathological staging in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332(7):429-35.
- Huang X, Pateromichelakis S, Hills A, Sherriff M, Lyons A, Langdon J, et al. p53 mutations in deep tissues are more strongly associated with recurrence thanmutation-positive mucosal margins. Clin Cancer Res. 2007;13(20):6099-106.
- van Houten VM, Leemans CR, Kummer JA, Dijkstra J, Kuik DJ, van den Brekel MW, et al. Molecular diagnosis of surgical margins and local recurrence in head and neck cancer patients: a prospective study. Clin Cancer Res. 2004;10(11):3614-20.
- Singh J, Jayaraj R, Baxi S, Mileva M, Skinner J, Dhand NK, et al. Immunohistochemical expression levels of p53 and eIF4E markers in histologically negative surgical margins, and their association with the clinical outcome of patients with head and neck squamous cell carcinoma. Mol Clin Oncol. 2016;4(2):166-72.
- Carvalho AC, Kowalski LP, Campos AH, Soares FA, Carvalho AL, Vettore AL. Significance of molecular alterations in histologically negative surgical margins of head and neck cancer patients. Oral Oncol. 2012;48(3):240-8.
- Supic G, Kozomara R, Jovic N, Zeljic K, Magic Z. Prognostic significance of tumor-related genes hypermethylation detected in cancer-free surgical margins of oral squamous cell carcinomas. Oral Oncol. 2011;47(8):702-8.
- Roh JL, Westra WH, Califano JA, Sidransky D, Koch WM. Tissue imprint for molecular mapping of deep surgical margins in patients with head and neck squamous cell carcinoma. Head Neck. 2012;34(11):1529-36.
- Shaw RJ, Hobkirk AJ, Nikolaidis G, Woolgar JA, Triantafyllou A, Brown JS, et al. Molecular staging of surgical margins in oral squamous cell carcinoma using promoter methylation of p16(INK4A), cytoglobin, E-cadherin, and TMEFF2. Ann Surg Oncol. 2013;20(8):2796-802.