Research Article
Screening of the Cassia Fistula Phytochemical Constituents by UPLC-ESI-QTOF-MS2
Cecilia Castro-Lopez, Romeo Rojas and Guillermo Cristian Guadalupe Martínez-Avila*
Department of Chemistry and Biochemistry, Autonomous University of Nuevo León, Mexico
*Corresponding author: Guillermo Cristian Guadalupe MartínezÁvila, Department of Chemistry and Biochemistry Autonomous University of Nuevo León, School of Agronomy, 66050, General Escobedo, Nuevo León, México
Published: 01 Jun, 2018
Cite this article as: Castro-Lopez C, Rojas R, Martínez-
Avila GCG. Screening of the Cassia
Fistula Phytochemical Constituents
by UPLC-ESI-QTOF-MS2. Clin Oncol.
2018; 3: 1477.
Abstract
Cassia fistula leaves and their extracts are one of the most widely used herbal products and/
or dietary supplements in the world. A systematic study of the phytochemical compounds is
necessary to establish quality parameters. A UPLC-ESI-QTOF-MS2 method was used to obtain
chromatographic profiles for the compounds present in C. Fistula leaves. The method was used to
identify 12 glycosylated phenolic compounds including one lignan, two phenolic acids and nine
flavonoids in an aqueous leaf extract obtained under two extraction methods.
Keywords: Cassia fistula; Phytochemical compounds; UPLC-ESI-QTOF-MS2
Introduction
Many of the plants contain a variety of phytopharmaceuticals, which have found very important applications in the fields of agriculture, health, food and nutrition development [1]. Cassia fistula L. (Fabaceae, Caesalpinioideae), a very common plant known for its medicinal properties (antipyretic, analgesic, anti-inflammatory, and hypoglycemic effects) is native to India, the Amazon and Sri lanka and diffused in various countries including Mexico, China, Mauritius, East Africa, South Africa and West Indies [2,3]. Over the past few years, there has been an exponential growth in study of primary and secondary metabolite composition that may be responsible for majority of the ascribed biological effects of this plant [4]. However, there are not enough studies that provide sufficient knowledge to the elucidation of specific phytochemicals present in this plant material. Hence, the present study was aimed to detect bioactive compounds present on extracts of Cassia fistula leaves obtained by different extraction methods.
Materials and Methods
Chemicals
Acetonitrile, methanol, water, and formic acid were all LC-MS grade and purchased from Fisher
Scientific Chemicals (Fair Lawn, NJ, USA).
Preparation of Cassia fistula extracts
The powdered Cassia fistula leaves were successively extracted by a conventional solid-liquid
extraction (decoction), and the use of emerging technologies (microwave-assisted extraction) using
a solid: liquid ratio of 1:50 w/v. The conditions used for both extraction methods are those reported
in a previous study [5].
Phytochemical screening by UPLC-ESI-QTOF-M2
Qualitative identification of phytochemicals was carried out using a BEH PHENYL (2.1mm
x 100mm, 1.7μm; waters, UK) analytical column operated at 40 °C and the chromatographic
separation was performed using a mobile phase of solvent A: 0.1% (v/v) formic acid water and
solvent B: 100% acetonitrile, with a constant flow rate of 0.3 mL min-1 with a gradient elution.
While the full screen mass spectra detection (UHPLC system coupled to a quadrupole time-of-flight
orthogonal accelerated QTOF mass spectrometer) was carried out in the negative ion mode in a
mass range m/z of 50-1200 Da and using a capillary voltage of -3.5 kV and +4.0 kV. For more details,
the complete conditions used are reported in a previous study [5].
Results and Discussion
The LC-MS analysis of phytocompounds in leaf extracts of Cassia Fistula explored the presence
of various bioactive components. The identification of the phytocompounds was confirmed based
on the molecular mass and its fragmentation pattern. The results (presented in Table 1) show that
C. fistula contains 10 bioactive compounds which are extracted and
differ according to the different extraction method applied in our
study. The identified compounds include one lignan, two phenolic
acids and seven flavonoids.
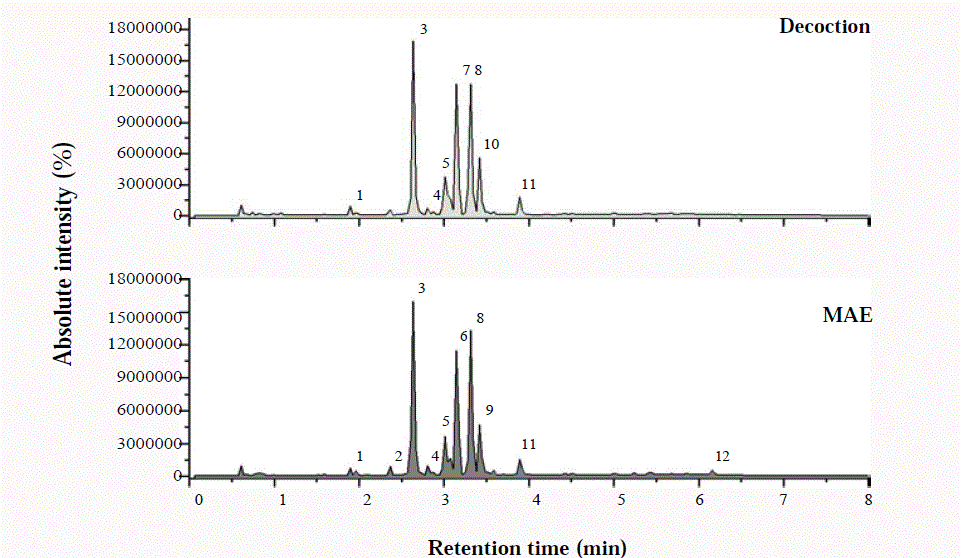
The LC-MS chromatogram of the 12 peaks of the compounds
detected was shown in Figure 1. Chromatogram LC-MS analysis
of the two extract of Cassia fistula (Decoction and MAE) showed
the presence of certain different peaks and the components
corresponding to the peaks were determined as follows. Peak 1
([M-H] - at m/z 417.0068) was identified as Syringaresinol. The
characteristic fragment ion at m/z 402.0354 is resulting from the
successive losses of two CH3 groups. It is necessary to emphasize
that seems to be the first report of the presence of Syringaresinol
in the leaves of Cassia Fistula. Peak 2 with a precursor ion at m/z
463.0265 produced a fragmentation ion at m/z 300.9823 ([M-H]- m/z
162], suggesting the presence of quercetin derivatives (Quercetin-Ohexoside).
Apigenin-6, 8-di-C-glycoside was assigned to Peak 3. This
compound exhibited a deprotonated molecule at m/z 592.9786 where
its MS2 spectrum produced a fragmentation ion at m/z 472.9887
which correspond for a fragmentation pattern of flavones di-Cglycoside
(apigenin as aglycone and two hexose moieties) [6]. Peak
4 presented a [M-H]- at m/z 563.0218, yielding a dominant fragment
at m/z 430.1055 (from the loss of the second glycosyl, a rhamnosyl)
suggesting that it could be a Kaempferol rhamnosyl xyloside. Peak 5
showed a base peak at m/z 561.0221 and displayed a fragmentation
pattern at m/z 439.0179 that could be attributed to a loss of glycoside
residues and was identified as Coumaric acid derivative. Apigenin-
6-C-pentoside-8-C-hexoside (Isomers) (Peaks 6-8) and Apigenin-
C-hexoside-O-pentoside (Peak 9) presented a pseudo-molecular
ion at m/z 562.98 and fragmentation patterns at m/z 443 and m/z
413, respectively, indicating the presence of a C-hexosyl unit typical
of the flavone asymmetric di-C-glycosides. Myricetin hexoside was
assigned to Peak 10 were the di-glycoside were determined based on
the detection of deprotonated ion at [M-H] - at m/z 478.9819 with a
major characteristic flavonol ion fragment at m/z 317.0013 [7]. Peak
11 had [M-H] - at m/z 576.9918 and was identified as type B dimer
of Proanthocyanidin [(epi) catechin-(epi) catechin] by comparison
of its fragmentation behavior with previous works ([M-H]- at m/z
425.0389) [8]. Finally, the presence of 3,4-di-O-caffeoylquinic acid
(Peak 12) was confirmed by its fragmentation pattern m/z 353.0241
→ 191, indicating the presence of a monocaffeoylquinic acid (loss of
caffeic acid moiety) [9]. The difference on composition in both extracts
(Decoction and MAE) can be attributed to microwave radiation that
has the property of transferring energy causing the instantaneous
superheating in vegetal sample promoting chemical transformations
which result in the organic synthesis of more compounds [10].
Table 1
Table 1
Phytochemical compounds detected and characterized in Cassia Fistula leaf extracts obtained by two extraction methods by using UPLC-Q/TOF-MS2
Figure 1
Figure 1
Base-peak chromatogram of phytochemical compounds in Cassia Fistula leaf extracts obtained by two extraction methods by using UPLC-Q/TOF-MS2.
Conclusions
This study provides evidence of the possible presence of several active compounds in the crude extracts from Cassia Fistula leaves and revealing the presence of a wide variety of flavonoids. Finally, the use of chromatography and mass spectrometry is particularly useful for the rapid characterization of unknown active compounds of an extract.
Acknowledgment
Authors thank to Mexican Council for Science and Technology (CONACYT) for the financial support given. Cecilia Castro-Lopez sincerely thanks M.Sc. Edgar T. Vazquez Ramos from Waters Corporation for his technical support.
References
- Pawar AV, Patil SJ, Killedar SG. Uses of cassia fistula linn as a medicinal plant. IJARnD. 2017;2(3):85-91.
- Kumar KA, Satish S, Sayeed I, Hedge K. Therapeutic uses of cassia fistula: Review. International Journal of Pharma and Chemical Research. 2017;3(1):38-43.
- Thirumal M, Surya S, Kishore G. Cassia fistula linn-pharmacogenetic, phytochemical and pharmacological review. Critical Review in Pharmaceutical Sciences. 2012;1(1):43-65.
- Bahorun T, Neergheen VS, Aruoma OI. Phytochemical constituents of cassia fistula. African Journal of Biotechnology. 2005;4(13):1530-40.
- Castro López C, Ventura Sobrevilla JM, González Hernández MD, Rojas R, Ascacio Valdés JA, Aguilar CN, et al. Impact of extraction techniques on antioxidant capacities and phytochemical composition of polyphenol-rich extracts. Food Chemistry. 2017;237:1139-48.
- Sakalem ME, Negri G, Tabach R. Chemical composition of hydroethanolic extracts from five species of the passiflora genus. Brazilian Journal of Pharmacognosy. 2012;22(6):1219-32.
- Dai X, Zhuang J, Wu Y, Wang P, Zhao G, Liu Y, et al. Identification of a flavonoid glucosyltransferase involved in 7-oh site glycosylation in tea plants (camellia sinensis). Sci Rep. 2017;7(1):5926.
- Spinola V, Castilho P, Pinto J. Identification and quantification of phenolic compounds of selected fruits from Madeira Island by HPLC-DAD-ESIMSn and screening for their antioxidant activity. Food Chemistry. 2014;173:14-30.
- Gouveia SC, Castilho PC. Characterization of phenolic compounds in Helichrysum melaleucum by high‐ performance liquid chromatography with on‐line ultraviolet and mass spectrometry detection. Rapid Commun Mass Spectrometry. 2010;24(13):1851-68.
- Hernández H, Leyva S. Using microwave radiation in the intramolecular cyclization and SNAr reaction of the synthetic pathway proposed for development of norfloxacin derivatives. Revista Mexicana de Ciencias Farmacéuticas. 2011;42(3):27-34.