Case Presentation
Solid Pseudo-Papillary Tumor of Pancreas - A Rare Case Report
Sood N and Jindal Y*
Department of Pathology, Deen Dayal Upadhyay Hospital, India
*Corresponding author: Nee Sood, Department of Pathology, Deen Dayal Upadhyay Hospital, C-5/102, Rohini, Sector 11, Delhi, India
Published: 13 May, 2018
Cite this article as: Sood N, Jindal Y. Solid Pseudo-
Papillary Tumor of Pancreas - A Rare
Case Report. Clin Oncol. 2018; 3: 1461.
Abstract
Solid Pseudopapillary Tumor (SPPT) of the pancreas is a rare tumor accounting for 1% to 3%
of exocrine pancreatic neoplasm. It typically affects mostly young women, often in their 20’s.
Present case is of 28 years old female patient with complaint of abdominal pain. Her radiological
investigation showed large retroperitoneal mass, measuring 10 cm × 9 cm × 8 cm with multiple small
central? Necrotic foci origin of this mass could not be ascertained. FNA done from retroperitoneal
mass under USG-guidance showed few atypical cells in clusters and scattered singly. These cells
showed moderate anisonucleosis, indistinct nucleoli and abundant cytoplasm. Few intracellular
and extracellular hyaline globules (PAS positive) present. Some cells showed coarsely vacuolated
cytoplasm. On this basis, differentials considered were SPPT, adrenocortical carcinoma, renal
cell carcinoma and rhabdoid tumor. Pancreatic mass along with pancreatic tail and spleen was
excised. According to cytological, radiological, gross, histological and immunological findings, final
diagnosis of solid pseudopapillary neoplasm of the pancreas was made. This case is of interest due
to its rarity, presence of hyaline globules and cytoplasmic vacuoles in microscopy, new molecular
markers and good prognosis of patients.
Keywords: Hyaline globules; Cytoplasmic vacuoles; Good prognosis; Pancreas
Case Presentation
A 28 years old female patient presented in surgery department with complaint of abdominal
pain. Her abdominal CT was advised and showed large heterogeneous hyperdense retroperitoneal
mass measuring 10 cm × 9 cm × 8 cm with multiple small central necrotic foci. Origin of this mass
could not be ascertained (Figure 1). FNA done from retroperitoneal mass under USG- guidance
showed mainly hemorrhage along with few atypical cells in clusters, pseudorosettoid pattern,
scattered singly and arranged around capillaries and thick blood vessels. These cells showed moderate
anisonucleosis, indistinct nucleoli and abundant cytoplasm. Few intracellular and extracellular
hyaline globules (PAS positive) were present.
Some cells showed coarsely vacuolated cytoplasm (PAS negative) (Figure 2). Our case didn’t
show prominent nucleoli, nuclear grooving and cercariform cells. Mitosis was infrequent.
According to cytological findings, differentials considered were SPPT, adrenocortical carcinoma,
renal cell carcinoma and rhabdoid tumor (Table 1). Subsequent MRI study showed pancreas as
the origin of this solid cystic mass (Figure 1). Biochemical markers were done (Amylase- 69U/L,
and Lipase- 98U/L). Distal pancreatectomy along with mass and spleen was excised. Gross showed
a well-defined pancreatic mass measuring 10 cm × 9 cm × 8 cm with pancreatic tail measuring 4
cm at one site. Cut surface of which was variegated, fleshy and showed greyish-white solid, cystic
and few hemorrhagic areas. Sections showed capsulated tumor mass showing tumor cells arranged
in mainly micro- and macro acinar pattern and focal pseudopapillary pattern. Cells showed mild
anisocytosis, round to oval nuclei, finely stippled chromatin, nuclear grooves, indistinct nucleoli and
eosinophilic cytoplasm. Intracellular and extracellular hyaline globules were present (PAS positive)
(Figure 3). Mitosis was insignificant. IHC showed beta catenin and cyclinD1 positive, CD10 focal
cytoplasmic positive, PR focal nuclear positive, DOG1 focal cytoplasmic and membranous positive
and CK7, CD56, CD117, chromogranin negative and Ki-67- Very low (Figure 4). Multiple sections
examined from spleen along with hilar vessels were free from metastasis. On the basis of cytological,
gross, histological and IHC findings, final diagnosis of Solid pseudopapillary tumor of the pancreas
was made.
Discussion
SPPT of the pancreas is a rare tumor and accounts for 1% to 3% of exocrine pancreatic neoplasm
of non-endocrine pancreatic neoplasms [1]. It typically affects mostly
young women, often in their 20’s [2]. Unlike other pancreatic tumor
it is not associated with any clinical syndrome. SPPT is also named
as ‘papillary cystic neoplasm’ and ‘Frantz’s tumor’. SPPT is the most
recent name advocated by the WHO [2]. Previously it has been called
by various other names as ‘solid and cystic’, ‘solid and papillary’,
‘cystic and papillary' and 'papillary-cystic' [3]. This plethora of names
is due to unknown origin of tumor as it lacks clear evidence of ductal,
acinar or endocrine differentiation. Recent studies have shown no
correlation between malignant potential with age and sex [1].
The patients with SPPT usually present as abdominal pain, nausea,
vomiting and palpable abdominal mass. Rarely, patients may present
with intestinal occlusion, jaundice, pancreatitis or traumatic rupture
with haemoperitoneum [1]. In present case patient present with
abdominal pain and mass was detected during general examination.
SPPT can be found anywhere in the pancreas.
Reported that the most common location of the tumor is the tail
of the pancreas (35.9%), then the head (34%), the body (14.8%) and
lastly the neck (1.01%) [4].
Radiologically, SPTs have a wide range of appearance from
solid to cystic, but a well encapsulated mass, with solid and cystic
component has been identified as a typical imaging finding of SPPT
[5]. Nakeeb AE et al. proved no difference in benign and malignant
component in pattern of calcification and solid and cystic proportion
of tumor and pancreatic duct dilatation [1]. Pancreatic masses may
mimic large non-pancreatic lesions on CT imaging which is a known
diagnostic pitfall as in this case [6]. Characteristic cytology is presence
of scattered intact papillary structures with delicate fibrovascular
cores as well as single and small loose clusters. Fibrovascular cores
may contain metachromatic material. Cells have delicate, finely
granular cytoplasm with frequently grooved nuclei and finely stippled
chromatin. Background may contain myxoid material [7]. Mitosis is
rare. Few studies shows the presence of hyaline globules that may be
seen extracellularly and intracellularly [7,8] and few of the studies
does not describe their presence [9,10].
Jahangir et al. described cercariform cells in their study that look
like cells with cytoplasmic tails and have been detached from the
fibrovascular cores during aspiration or smearing [11]. Present study
didn’t show these forms.
Recent study has shown importance of vacuolated cytoplasm
in differentiating SPPT from Pancreatic Neuroendocrine Neoplasm
(PET) that could be a close mimicker as aspirates from both tumors
show moderate to high cellularity, low N:C ratio, nucleoli along with
plasmacytoid appearance [10]. Mehta N et al. described the presence
of pseudorosette pattern in their study which is again a point to
differentiate it from PET [9].
Intraductal papillary carcinoma show columnar cells with variable
nuclear anaplasia, irregular chromatin, and prominent nucleoli and
should also be rule out. Thick glistening and viscid mucus material,
almost always present in intraductal papillary mucinous tumor, is an
important feature that distinguishes this neoplasm from SPPT. In
case of ductal adenocarcinoma smears show cells arranged in three
dimensional clusters, microglandular pattern and occasional true
papillary fragments with obvious features of malignancy [11].
Pancreatoblastoma, a tumor of childhood lacks the
pseudopapillary pattern and fibrovascular stalks with myxoid stroma
and is consistently negative for vimentin and positive for pancreatic
enzymes, that distinction it from SPPT. Due to presence of varied
morphology and hyaline globules, differential diagnosis considered
were SPPT, adrenocortical carcinoma, renal cell carcinoma and
rhabdoid tumor as the origin of tumor could not be made by CT.
Grossly, it is usually large (mean 9 cm) and encapsulated, with
variable solid and cystic areas, as well as hemorrhagic and necrotic
foci. It has been studied that the cavities in SPPTs are not 'true' cysts as
they lack epithelial lining but rather represent a necrotic/degenerative
process [3].
On histopathology, tumor contain pseudopapillae with hyalinized
fibrovascular cores lined by several layers of bland fragile epithelial
cells with clear to eosinophilic cytoplasm with variable mucinous
changes within the core and intracytoplasmic PAS + diastase resistant
hyaline globules. Pseudopapillary pattern is due to solid nests minus
cells degenerating away from the small vessels, resembling rosettes
in cross section. Ren et al. showed solid areas are composed of sheets
and cords of discohesive tumor cells with extensive microcystic space
formation and apparent hyaline degeneration in the stroma, features
that is similar to present case [12]. Nuclei are round/oval with finely
stippled chromatin, nuclear grooves, indistinct nucleoli and few
mitoses. Tumor cells infiltrate without any stromal reaction. The
cystic areas often contain blood, necrotic debris, clusters of foamy
macrophages, giant cells along with stromal degeneration in the form
of myxoid change and calcification. These changes are mainly seen in
large tumors and represents inflammatory response due to necrotic
change.
A clear cell variant of SPPT has been described where the
tumor cells have clear cytoplasm. This has been attributed to
distended mitochondria and endoplasmic reticulum. This creates a
diagnostic challenge in distinguishing it from other clear cell tumors
of the pancreas such as metastatic renal cell carcinoma, ectopic
adrenocortical nodules, clear-cell variant of pancreatic endocrine
neoplasm, and ductal adenocarcinoma. IHC is helpful in making a
definitive diagnosis in such cases [13].
SPPT was classified according to the WHO as either SPPT with
an uncertain potential for malignancy or Solid Pseudopapillary
Carcinoma (SPC). Study have shown that unequivocal perineural
invasion, angioinvasion, deep invasion into the surrounding tissue
or distant metastasis indicate malignant behavior, and such lesions
should be classified as SPC [2], whereas other studies have shown
non-specific malignant behavior and malignant potential could
not be completely excluded even in the absence of pathological
feature [14] Papavramidis et al. concluded that 20 % of SPPT shows
malignant behavior [4]. Almost all tumors have mutations in exon
3 of the beta-catenin gene, which causes abnormal immunostaining
patterns for beta-catenin (nuclear and cytoplasmic, compared to
membranous staining in normal pancreas) and overexpression of
cyclinD1. Present case was positive for beta-catenin (Cytoplasmic
and nuclear positivity) and Cyclin D1 (nuclear positivity). Previous
studies showed APC/β-catenin pathway and cyclin-D1 alterations
uniformly present in almost >90% of this tumor [15] Recent studies
have also shown nuclear localization of E-cadherin with absence of
membranous and cytoplasmic localization, which may account for
the dyscohesive nature and cystic formation of the tumor [16].
Tumor cells are also immunoreactive for non-specific markers
like CD 10, CD56, CD99, progesterone receptor, alpha-1-antitrypsin
and vimentin [1]. Synaptophysin and NSE are commonly positive,
however, chromogranin, the most specific endocrine marker is
typically negative, hence differentiation from neuroendocrine tumor
could be made. As SPPT occur more commonly in female and express
progesterone receptor, suggesting the role of female hormone in the
evolution of these tumors. A recent hypothesis mentions that SPPT
arise from pancreatic pluripotent stem cells [12]. Present case showed
focal cytoplasmic positivity for CD10 and negative for CD99.
Recently, c-kit (CD117) expression was detected in most cases of
SPPTs. The molecular mechanism of KIT over expression in these
tumors remains unknown. Immunohistochemical staining of KIT has
been demonstrated in many types of tumors that do not or only very
rarely harbor KIT mutations. The absence of KIT mutations in these
neoplasms suggests that there is other mechanism than activating
mutations to over express KIT protein. One possible mechanism
is gene dose effect as a result of increased copies of KIT gene could
be result of c-kit overexpression [17]. Also recent literature had
shown the expression of DOG1 in SPPT [18]. As DOG1 is expressed
in centroacinar cells and also in SPPT, centroacinar cells could be
the cell of origin of this tumor. However, CD117 was negative and
DOG1was focally positive in present case.
SPPT has a low malignant potential and excellent prognosis, with
a high cure rate after resection. Even in the presence of disseminated
disease, the clinical course is usually favorable [19] with very low
reccurence rate of 8.3% [1]. Nakeeb et al. also described slightly
increased levels of tumor markers (CEA and CA19-9) in patients SPN
showing malignant change [1].
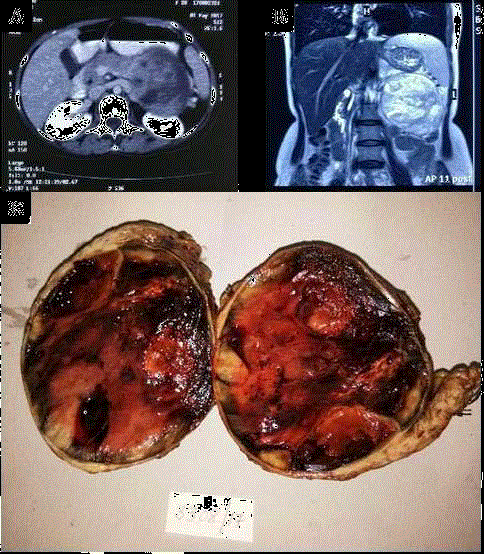
Figure 1
Figure 1
CT & MRI: Axial image on contrast CT & T2 coronal image on MRI
shows well defined lesion with heterogenous enhancement seen originating
from body and tail of pancreas with mass effect on kidney posteriorly and
stomach anteriorly.
Gross: Well circumscribed capsulated mass (10 cm × 9 cm × 8 cm) along
with tail of pancreas. Cut surface is fleshy with solid, cystic and hemorrhagic
areas.
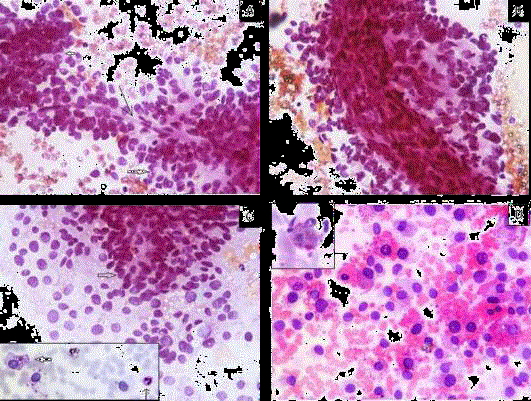
Figure 2
Figure 2
A) Perivascular arrangement of tumor cells with pseudo rosette
formation (solid arrow) around capillary (arrow), (PAP × 400).
B) Arrangement around thick blood vessel, (PAP × 400).
C) Tumor cells scattered singly with naked nuclei as well as pseudo rosette
formation (solid arrow) (PAP × 400). [Inset: PAS negative intracytoplasmic
vacuoles (solid arrow) with internal positive control (arrow)].
D) Extracellular and intracellular hyaline globules (H&E × 400) [Inset: PAS-D
positive].
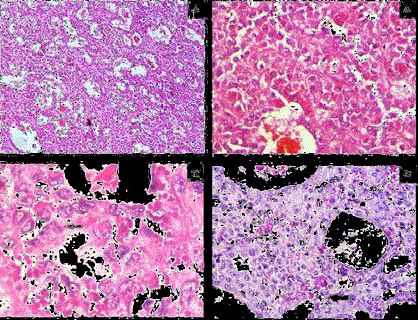
Figure 3
Figure 3
A) Tumor cells arranged in predominantly micro- and macro-acinar
pattern. (H&E × 100).
B) Tumor cells show mild anisocytosis, round to oval nuclei, finely stippled
chromatin, indistinct nucleoli and eosinophilic cytoplasm (H&E × 400).
C) Few tumor cells show grooving (solid arrow) (H&E × 1000).
D) PAS-D positive intracellular (arrow) and extracellular (solid arrow) hyaline
globules (PAS-D × 400).
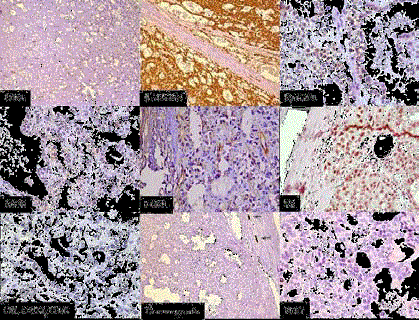
Figure 4
Figure 4
IHC: CD31 highlightling vascularity with lack of papillary
configuration, β- catenin- Nuclear and cytoplasmic positivity, Cyclin
D1- Nuclear positivity, CD10- Focal cytoplasmic positive, DOG1 - Focal
cytoplasmic and membranous positive, PR - Focal nuclear positive, CK7,
CD117 , CD56-Negative, Chromogranin- Negative with internal positive
control, Ki-67- Very low.
Conclusion
This case is of interest due to its rarity, presence of hyaline globules as well as cytoplasmic vacuoles in cytology, new molecular markers and good prognosis of patients.
References
- Nakeeb AE, Wahab MA, Elkashef WF, Azer M, Kandil T. Solid pseudopapillary tumour of the pancreas: Incidence, prognosis and outcome of surgery (single center experience). Int J Surg. 2013;11(6):447-57.
- Klöppel G, Lüttges J. WHO-classification 2000: Exocrine pancreatic tumors. Verh Dtsch Ges Pathol. 2001;85:219-28.
- Klimstra DS, Wenig BM, Heffess CS. Solid-pseudopapillary tumor of the pancreas: A typically cystic tumor of low malignant potential. Semin Diagn Pathol. 2000;17(1):66-81.
- Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg. 2005;200(6):965-72.
- Yin Q, Wang M, Wang C, Wu Z, Yuan F, Chen K, et al. Differentiation between benign and malignant solid pseudopapillary tumor of the pancreas by MDCT. Eur J Radiol. 2012;81(11):3010-8.
- Lawler LP, Horton KM, Fishman EK. Peripancreatic masses that simulate pancreatic disease: spectrum of disease and role of CT. RSNA. 2003;23(5):1117-31.
- Bellizzi AM, Stelow EB. Pancreatic cytopathology a practical approach and review. Arch Pathol Lab Med. 2009;133(3):388-404.
- Uppin SG, Hui M, Thumma V, Challa S, Uppin MS, Bheerappa N, et al. Solid-pseudopapillary neoplasm of the pancreas: A clinicopathological and immunohistochemical study of 33 cases from a single institution in Southern India. Indian J Pathol Microbiol. 2015;58(2):163-9
- Mehta N, Modi L, Patel T, Shah M. Study of cytomorphology of solid pseudopapillary tumor of pancreas and its differential diagnosis. J Cytol. 2010;27(4):118-22.
- Jhala N, Siegal GP, Jhala D. Large, clear cytoplasmic vacuolation: An under-recognized cytologic clue to distinguish solid pseudopapillary neoplasms of the pancreas from pancreatic endocrine neoplasms on fineneedle aspiration. Cancer. 2008;114(4):249-54.
- Jahangir S, Loya A, Siddiqui MT, Noreen N, Yusuf MA. Accuracy of diagnosis of solid pseudopapillary tumor of the pancreas on fine needle aspiration: A multi-institution experience of ten cases. Cyto J. 2015;12:29.
- Ren Z, Zhang P, Zhang X, Liu B. Solid pseudopapillary neoplasms of the pancreas: Clinicopathologic features and surgical treatment of 19 cases. Int J Clin Exp Pathol. 2014;7(10):6889-97.
- Hav M, Lem D, Chhut SV, Kong R, Pauwels P, Cuvelier C, et al. Clear-cell variant of solid-pseudopapillary neoplasm of the pancreas: A case report and review of the literature. Malays J Pathol. 2009;31(2):137-41.
- Tang LH, Aydin H, Brennan MF, Klimstra DS. Clinically aggressive solid pseudopapillary tumors of the pancreas: A report of two cases with components of undifferentiated carcinoma and a comparative clinicopathologic analysis of 34 conventional cases. Am J Surg Pathol. 2005;29(4):512-9.
- Abraham SC, Klimstra DS, Wilentz RE, Yeo CJ, Conlon K, Brennan M, et al. Solid-pseudopapillary tumors of the pancreas are genetically distinct from pancreatic ductal adenocarcinomas and almost always harbor betacatenin mutations. Am J Pathol. 2002;160(4):1361-9
- El-Bahrawy MA, Rowan A, Homcastle D, Tomlinson I, Theis BA, Russell RC, et al. E-cadherin/catenin complex status in solid pseudopapillary tumor of the pancreas. Am J Surg Pathol. 2008;32(1):1-7.
- Cao D, Antonescu C, Wong G, Winter J, Maitra A, Adsay NV, et al. Positive immunohistochemical staining of KIT in solid-pseudopapillary neoplasms of the pancreas is not associated with KIT/PDGFRA mutations. Mod Pathol. 2006;19(9):1157-63.
- Bergmann F, Andrulis M, Hartwig W, Penzel R, Gaida MM, Herpel E, et al. Discovered on gastrointestinal stromal tumor 1 (DOG1) is expressed in pancreatic centroacinar cells and in solid-pseudopapillary neoplasms-- novel evidence for a histogenetic relationship. Hum Path. 2011;42(6):817-23.
- Martin RC, Klimstra DS, Brennan MF, Conlon KC. Solid-pseudopapillary tumor of the pancreas: A surgical enigma?. Ann SurgOncol. 2002;9(1):35-40.