Case Report
Pediatric Extra Renal Reno cellular Carcinoma with Xp11 Translocation Responding to Sunitinib, Everolimus and Metronomic Chemotherapy
István Szegedi*, Gábor Méhes, Imre Gáspár, Tamás Csonka, István Csízy and Csongor Kiss
Department of Pediatrics, University of Debrecen, Hungary
*Corresponding author: István Szegedi, Department of Pediatrics, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary
Published: 20 Apr, 2018
Cite this article as: Szegedi I, Méhes G, Gáspár I, Csonka
T, Csízy I, Kiss C. Pediatric Extra
Renal Reno cellular Carcinoma with
Xp11 Translocation Responding to
Sunitinib, Everolimus and Metronomic
Chemotherapy. Clin Oncol. 2018; 3:
1458.
Abstract
Background: Fewer than 2% of renal cell carcinomas (RCC) develop in childhood, making up less
than 0.1-0.3% of all pediatric tumors and 2.6% of renal neoplasms. The 2004 WHO classification
defined RCC with Xp11.2 aberration as a separate entity accumulating characteristically in
childhood. Ectopic development of pediatric renal neoplasms is rare and there are no data in the
literature about the extra renal origin of RCC.
Case Presentation: We describe the case of a 16-year old boy with an extra renal RCC showing
Xp11.2 translocation demonstrated by FISH. The tumor exhibited a favourable response to a
combination of targeted treatment with sunitinib and everolimus in association with metronomic
chemotherapy.
Conclusion: The novelty of the reported case, in addition to the extra renal origin of the tumor, is
the favourable response to a combination of sunitinib, everolimus and metronomic chemotherapy
resulted in a major partial control of advanced RCC accompanied by a 15 months survival from
diagnosis without major toxicities.
Keywords: Renal cell carcinoma; pediatric; Extra renal; Xp11 translocation; Targeted therapy; Metronomic treatment
Background
Renal cell carcinoma (RCC) is a rare form of childhood cancer representing fewer, than 0.1- 0.3% of all tumors and 2.6% of renal neoplasms in the pediatric population. Less than 2% of all RCC cases occurs in pediatric patients, with a highest incidence between 15-19 years [1-2]. The 2004 WHO classification defined RCC with Xp11.2 aberration as a separate entity accumulating characteristically in childhood.RCC associated with Xp11.2 translocation characteristically affects children; however, adult cases may outnumber pediatric cases due to a much higher overall incidence in the adult population [3-4]. The most frequent pediatric renal neoplasm is Wilms tumor which rarely may originate from an extra renal ectopic nephrogenic rest [5-6]. There is no data, however in the literature about the extra renal origin of RCC with classical clear cell morphology. Targeted therapies with broad spectrum tyrosine kinas inhibitors (TKI), agents interfering with the effects of vascular endothelial growths factor (VEGF) and inhibitors of the Mammalian Target of Rapamycin (MTOR) have been shown superior to either immunotherapy or to placebo treatment in randomized phase III trials designed for adult patients with RCC [7]. The above agents have not been registered for use in children and pediatric experiences with the treatment of RCC are restricted to sporadic case reports only [8].Combined oral metronomic bio differentiating anti angio genetic treatment /COMBAT/ proved promising in a series of advanced refractory pediatric malignancies [9]. However, there are only limited data on the efficacy and safety of metronomic therapy in RCC [10]. Here we report, for the first time, on the application of the multi targeted oral TKI sunitibib in combination with the MTOR inhibitor everolimus and metronomic chemotherapy resulting in major partial response in a 16 -year old boy with an extensive extra renal RCC.
Case Presentation
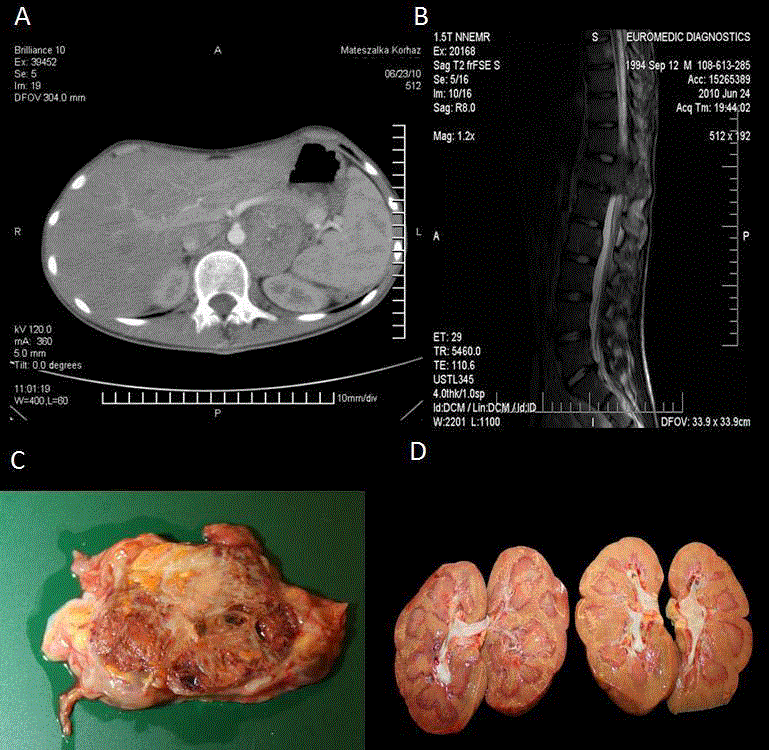
A16-year old Caucasian male was referred to the Department of Pediatric Hemotology- Oncology, University of Debrecen, in June, 2010 because of lower limb pain for the last six months, fatigue, anorexia, and significant weight loss. On referral he was in a poor general condition, was significantly underweight (height: 175 cm; 50-75 percentile; weight: 45 kg ;< 3 percentile).Abdominal ultra sonography showed a 5.5 cm large in homogeneous lesion at the left suprarenal region containing areas of calcification. Contrast enhanced abdominal computed tomography scan confirmed the same mass, which was completely separated from the left kidney (Figure 1A). There were enlarged paraaortic lymph nodes present, and an additional destructive 10 cm large lesion on the right side of the pubic bone, with wide muscular infiltration, and an epidural mass with intra spinal invasion at the L2region. Magnetic resonance imaging showed a left par vertebral necrotic mass spreading behind the left renal vein and artery, a 10 mm enhancing mass at the corpus of ThVIII, a compression of ThXII resulting in the narrowing of the spinal canal, and another mass compressing the cone at the level of LII (Figure 1B). In addition, there was an infiltration of the sacral bones in both sides. Tc-99m metilen-diphosphonat bone scan revealed an additional right sided infiltration of the hip joint. I-131 MIBG scan did not show enhancement of the tracer. Initial laboratory tests revealed moderate anaemia (86g/L), a normal Neuron Specific Enolase (NSE) level and elevated plasma noradrenalin and urinary dopamine levels. Neurosurgery was performed to achieve decompression at the level of ThXII. The suprarenal location of the primary lesion and the elevated catecholamine level prompted us to start an urgent chemotherapeutic treatment according to the SIOP High Risk Neuroblastoma protocol [11]. Despite treatment, progressive disease was seen. In the meantime, histological evaluation revealed a necrotic and infiltrative clear cell carcinoma, which was positive for Oil-red-O, CD10, Bcl2, NSE and negative for Pan-Cytokeratin (PCK), GFAP, chromogranin, synaptophysin, desmin, inhibit and nest in, a typical morphological and immune phenotypic pattern suggesting the diagnosis of RCC with Xp11 translocation (Figure 2). Unresponsiveness to conventional chemotherapy was consistent with the diagnosis of RCC therefore; we started sunitinib treatment (1x50mg in 4 week-cycles, 6 weekly) and a complex bone supportive therapy (sodium clodronate, vitamin D) in September, 2010. Later, sunitinib treatment was supplemented with oral cyclophosphamide (2x25 mg/day) and isotretinoin as a part of COMBAT metronomic therapy [9]. The patient experienced an8-months-long period of stable disease. Due to progression in June 2011mTOR inhibitor everolimus has been added to the ongoing therapy (2x10 mg/day). After a week of treatment there was a prominent decrease in tumor volume as checked by physical examination with an almost complete disappearance of the huge inguinal resistance. In August, 2011a new histological sample was taken from this inguinal region. Microscopy showed a necrotic and haemorrhagic tissue mass without signs of viable RCC infiltration. In September, 2011 a Tc 99m bone-scan revealed major regression of the former metastatic regions without signs of osteoblastic activation. Later in September, 2011 the patient developed an excessive pneumonia and succumbed to death despite combined antibiotic treatment and mechanical ventilation. Autopsy findings were in agreement with the previous images including the suprarenal localisation of the primary lesion which was independent from the kidney. (Figure 1 C, D). Post-mortem histology confirmed the diagnosis of clear cell cancer equivalent in every regard of RCC. Residual tumor masses contained large areas of necrosis and hemorrhage intermixed with focal scarring. Intact tumor was present only in small foci.
Figure 1
Figure 1
The horizontal view of the tumor on the left par renal region. A) and the compressing mass of the spinal cord at LII level. B) Macroscopic appearance of the residual tumor removed during autopsy from the left par vertebral/suprarenal region of the retro peritoneum showing heterogeneous tissue composition including focal necrosis haemorrhage and consecutive fibrosis due to treatment response. C) Both kidneys were intact and lacked any evidence of tumor involvement during autopsy. D).
Methods
Histological examination was performed following fixation in 4.0% buffered formaldehyde and paraffin embedding as usual. Four-micrometer-thick sections were prepared and stained with hematoxilin-eosin.Immunohistochemistry was carried out with the Bond Max immune stainer (Leica, Wetzlar, Germany) using a panel of antibodies for PCK, CD10, Bcl2, NSE, GFAP, chromogranin, synaptophysin, desmin, inhibin and nest in (Novocastra, Newcastle, Great Britain). FISH analysis was performed on the few residual tumor cell nests on the formalin fixed paraffin embedded sections. The TFE3 Xp11 break apart probe kit (Kreatech Diagnostics, Amsterdam, The Netherlands)was applied to detect the translocation involving the TFE3 transcription factor gene region at Xp11. A break was defined as a separate red (centromeric) and green (telomeric) signals demonstratinga TEF3 rearrangement in tumor cells in contrast to red-green signals representing the normal TEF3 gene. 72/100 cell nuclei were identified with the break apart FISH signal pattern.
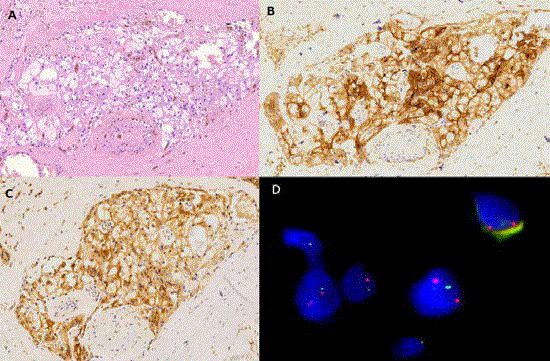
Figure 2
Figure 2
Conventional HE staining presented infiltrative foci of an atypical clear cell tumor with significant anisocytosis and a mild tendency of tubule formation. A) IHC for CD10. B) and bcl-2. C) Revealed as positive in the tumor cells while pan-cytokeratin expression (not shown) could not be stated (x200 magnification).
Fluorescence in situ hybridization analysis of the Xp11 region tumor cell nuclei. D) Specific probes flanking the centromeric (red signal) and telomeric (green signal)
parts of the TFE3 locus at Xp11displayed a clear dissociation pattern indicative of the breakage between the two binding sites (x1000 magnification).
Conclusions
In spite of major advances in therapy, metastatic RCC is still considered an incurable disease. In adults, 25-30% of patients present with metastases at time of diagnosis [12].In contrast to many other forms of cancer, introduction of combined chemotherapy and immunotherapy did not change the grave prognosis of RCC which proved a constitutionally chemoresistant malignant disease. Targeted therapy aimed at involved signalling pathways has become first line treatment [12]. Treatments with multi kinase inhibitors and MTOR inhibitors resulted usually in Disease Stabilization (SD) and elicited partial responses, whereas complete responses were rarely reported [13]. Several ongoing clinical studies investigate the efficacy of different combinations of targeted agents; however, none of the experimental combinations have yet been introduced in routine clinical practice. Experiences with RCC occurring in children are still exceptional. COMBAT is a feasible and effective treatment option for patients with refractory/relapsing malignancies characterized by a low toxicity profile; however, it has not been applied in pediatric RCC [9]. In this report we presented an aggressive extra renal clear cell tumor characterized by Xp11 translocation in a 16-year old boy, which was equivalent to RCC in all aspects [4].To the best of our knowledge this is the first extra renal case of RCC, regardless of the age, that has been reported in the literature. Detailed morphological analysis could not identify any other tissue component to the clear component which was moderately differentiated forming tubular arrangement (Figure 2A). The characteristic immune profile of the cells was rather homogenous inducing CD10 and bcl-2 immune positivity next to negativity for general mesenchymal and neurogenic markers (Figure 2B). FISH analysis performed on the few residual tumor cell nests demonstrated the duplication of the X chromosome due to two copies of both the green and the red FISH signals. The characteristic break apart Xp11 translocation pattern was stated on both (supernumerary) X chromosomes (Figure 3) as green and red signals completely separated in the majority (72%) of cell nuclei. The novelty of the reported case, in addition to the extra renal origin of the tumor, is the favourable response to a combination of sunitinib, everolimus and metronomic chemo therapy. The oral multi targeted receptor tyrosine kinase inhibitor sunitinib displays a 31% objective response rate in metastatic RCC patients resulting in a median progression free survival from 5-11 months [14]. For patients with advanced, metastatic RCC who progressed on VEGF receptor tyrosine kinase inhibitor therapy, the MTOR inhibitor everolimus has been shown to prolong progression free survival vs. placebo from 1.9 months to 4.9 months. Unfortunately, combination of everolimus and sunitinib was associated with significant acute and chronic toxicities at standard doses and showed infrequent efficacy in clear cell RCC in an adult phase I trial [7]. Here we reported that combined use of targeted therapies combined with metronomic type of cytostatic treatment resulted in a major partial control of advanced RCC accompanied by a 15 months survival from diagnosis. No major toxicities were observed. The presented case may suggest possible merits of combining TKI-s and MTOR inhibitors with COMBAT in metastatic RCC. The death of the patient was independent from tumor progression as proven by the autopsy findings.
Consent
Written informed consent was obtained from the patient’s mother for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
List of Abbreviations
RCC –renal cell carcinoma; WHO – World Health Organization; FISH – fluorescence in situ hybridization; TKI – tyrosine kinase inhibitor; VEGF – vascular endothelial growths factor; MTOR – mammalian target of rapamycin; COMBAT – combined oral metronomic bio differentiating anti angio genetic treatment; MIBG – metaiodibenzyl-guanidine; NSE – neuron specific enolase; SIOPInternational Society of Pediatric Oncology; CD10 – cell differentiation antigen; Bcl2 – B-cell lymphoma 2; PCK – pan-cytokeratin; GFAP – glial fibrillary acidic protein; TFE3 – transcription factor E3; SD – stable disease.
Contributors’ Statement
István Szegedi: Dr. Szegedi designed and organized the main
diagnostic procedure of the case, planned and conducted the
particular treatment steps and conceptualized and designed this
case report, drafted the initial manuscript, and approved the final
manuscript as submitted.
Gábor Méhes: Prof. Méhes coordinated the histological and
molecular diagnostic investigations and the autopsy, and reviewed
and critically revised the manuscript, and approved the final
manuscript as submitted.
Imre Gaspar: Dr. Gaspar helped to carry out the main steps of
the treatment, and reviewed the manuscript, and approved the final
manuscript as submitted.
Tamás Csonka: Dr. Csonka organized and carried out the
histological, molecular diagnostic investigations and the autopsy,
and reviewed the manuscript, and approved the final manuscript as
submitted.
István Csízy: Dr. Csízy carried out the surgical invasive procedures
and performed all the sampling of the patient, and reviewed the
manuscript, and approved the final manuscript as submitted.
Csongor Kiss: Prof. Kiss supervised the diagnostic and the therapeutic
steps, critically reviewed and revised the manuscript, and approved
the final manuscript as submitted.
Acknowledgement
This work was supported by TÁMOP 4.2.2.A-11/1/KONV-2012- 0025 project which are implemented through the New Hungary Development Plan, co-financed by the European Social Fund.
References
- Ramphal R, Pappo A, Zielenska M, Grant R and Ngan BY. Pediatric renal cell carcinoma. Am J Clin Pathol.2006; 126(3): 349-64.
- Salehipour M, Rasekhi A, Vasei M, Hasanpour A. Renal cell carcinoma in a child. Saudi J Kidney Diseases Transplantion. 2009; 20(1): 124-6.
- Lopez-Beltran A, Scrapelli M, Montironi R and Kirkali Z. WHO classification of the renal tumors of the adults. Eur Urol. 2006; 49(5): 798-805.
- Ross H. Argani P: Xp11 translocation renal cell carcinoma. Pathology. 2010; 42(4): 369-73.
- Taguchi S, Shono T, Mori D and Horie H. Extrarenal Wilms tumor in children with unfavorable histology: a case report. J Ped Surg. 2010; 45(9): E19-22.
- Cooke A, Deshpande AV, Hei ER, Kellie S, Arbuckle S and Cummis G: Ectopic nephogenic rests in children: the clinicosurgical implications. J Ped Surg. 2009; 44(12): E13-6.
- Molina AM, Feldman DR, Voss MH, Ginsberg MS, Baum MS et.al. Phase 1 trial of everolimus plus sunitinib in patients with metastatic renal cell carcinoma. Cancer. 2012; 118(7): 1868-76.
- Chowdhury T, Prichard-Jones K, Sebire NJ, Bier N, Cherian A, Sullivan Mo et.al. Persistent Complete Response After Single-agent Sunitinib Treatment in a Case of TFE Translocation Positive Relapsed Metastatic Pediatric Renal Cell Carcinoma. J Pediatr Hematol Oncol. 2013; 35(1): e1-3.
- Zapletalova D, André N, Deak L, Kyr M, Bajciova V, Mudry P et.al. :Metronomic chemotherapy with the COMBAT regimen in advanced pediatric malignancies: a multicenter experience. Oncology. 2012; 82(5): 249-60.
- Huijts CM, Santegoets SJ, Eertwegh AJ,Pijpers LS, Haanen JB, Gruijl T et.al. Phase I-II study of everolimus and low dose oral cyclophosphamide in patients with metastatic renal cell cancer. BMC Cancer. 2011; 11: 505- 12.
- Veal GJ, Nguyen L, Paci A, Riggi M, Amiel M, Valteau-Couanet D, et.al. Busulfan pharmacokinetics following intravenous and oral dosing regimens in children receiving high-dose myeloablative chemotherapy for high-risk neuroblastoma as part of the HR-NBL-1/SIOPEN trial. Eur J Cancer. 2012; 48(16): 3063-72.
- Rini BI. Vascular endothelial growth factor-targeted therapy in renal cell carcinoma: current status and future directions. Clin Cancer Res. 2007; 13(4): 1098-106.
- Coppin C, Kollmannsberger C, Le L, Porzsolt F, Wilt TJ: Targeted therapy for advanced renal cell cancer (RCC): a Cochrane systematic review of published randomised trials. BJU Int. 2011; 108(10): 1556-63.
- Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O et al:Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007; 356: 115-24.